Cidara Therapeutics Showcases New Findings on Innovative Drug-Fc Fusion Molecules at AACR's 2024 Meeting
Cidara Therapeutics, Inc., an enterprise specializing in biotechnological advancements, leverages its unique Cloudbreak technology to create innovative drug-Fc conjugate treatments. These therapies aim to enhance the quality of healthcare and provide life-saving options for patients battling severe illnesses. The firm has revealed plans to present a quartet of poster displays at the upcoming American Association for Cancer Research Annual Meeting. Among these, one presentation is identified as a late-breaking submission. The significant event is scheduled to take place from the 5th to the 10th of April, 2024, and will be hosted at the San Diego Convention Center, located in San Diego, California.
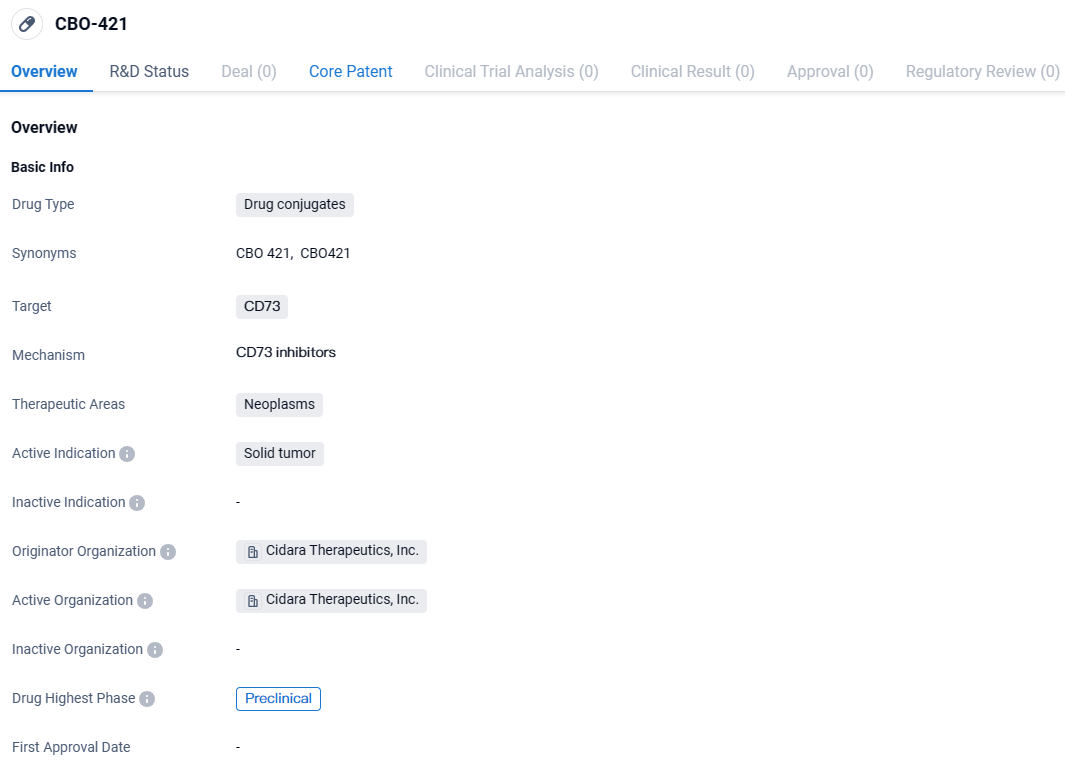
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The disclosed materials offer insights into the corporation’s multifaceted Fc-conjugate pharmaceutical that acts on CD73/PD-1, its drug focused on CCR5 known as DFC, and the prime DFC drug candidate CBO421, which is designed to target CD73 in the field of oncology.
"Our exploratory findings presented at the AACR conference this year underscore the potential our pioneering or superior DFCs hold in potentially enhancing therapies for diverse oncological conditions," remarked Jeffrey Stein, Ph.D., who holds the roles of president and CEO at Cidara.
"Our innovative Cloudbreak platform has rapidly brought forth DFC candidates showing efficacy in preclinical models for a range of cancers looking at precise pathological targets. This time, we've unveiled findings from our inaugural venture into the CCR5 DFC program, revealing significant effectiveness as a stand-alone therapy in laboratory mice with colorectal cancer.
The strategy of aiming at CCR5 could potentially increase the effectiveness of treatments involving immune checkpoint inhibitors, offering hope especially for patients who show no response to existing therapies. In addition, both our multi-targeting CD73/PD-1 DFC and CBO421, the lead drug in our CD73-targeting DFC lineup, continue to show promising results in fighting cancer, and we are enthusiastic about propelling these candidates closer to clinical application," continued Jeffrey Stein.
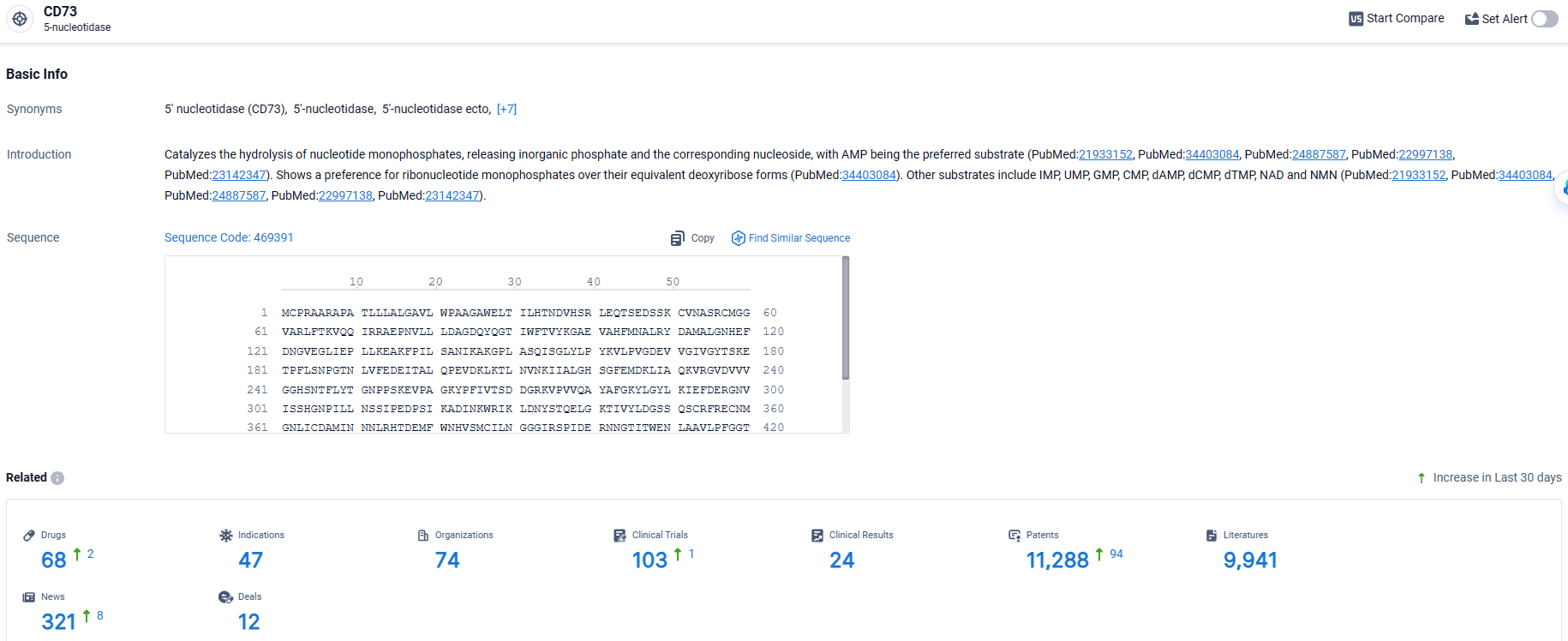
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 8, 2024, there are 68 investigational drugs for the CD73 target, including 47 indications, 74 R&D institutions involved, with related clinical trials reaching 103, and as many as 11288 patents.
CBO-421 is a drug conjugate being developed by Cidara Therapeutics, Inc. It targets CD73, a protein involved in neoplasms, specifically solid tumors. Currently in the preclinical phase, CBO-421 shows potential as a treatment option for solid tumors, but further research is needed to determine its effectiveness and safety in humans.