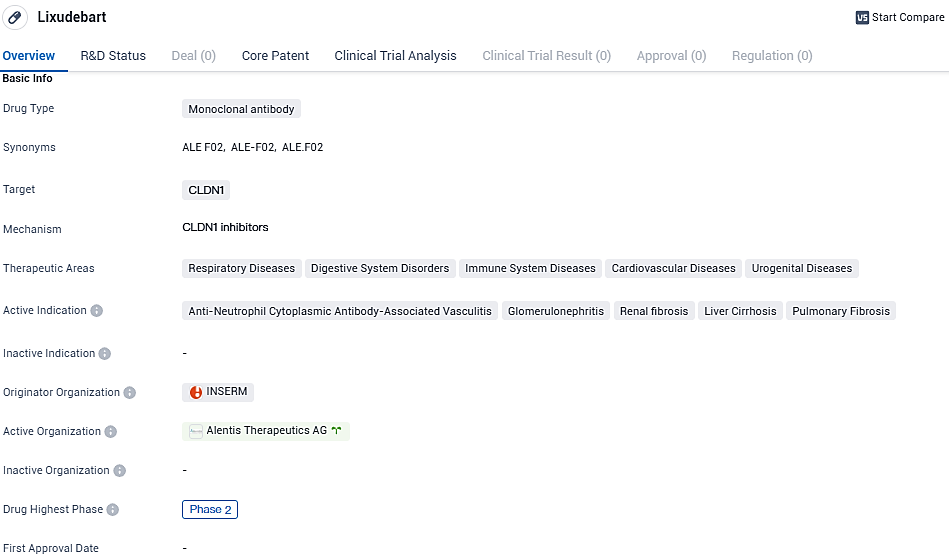
First ANCA-related vasculitis patient with rapid kidney involvement joins and receives treatment in Alentis Therapeutics’ Phase II Lixudebart (ALE.F02) trial
Alentis Therapeutics, a biotech firm in the clinical phase that focuses on the creation of therapies for tumors positive for Claudin-1 and fibrotic conditions in organs, has reported the initial dosing of a participant in a Phase 2 clinical study for lixudebart (formerly named ALE.F02). This investigational therapeutic antibody, which targets Claudin-1, seeks to address the issue of fibrosis in various organs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
A scientific trial, known as a Phase 2 trial, has been designed to appraise the investigational medication, lixudebart. This trial is critical in determining the drug's safety profile, how it's tolerated by patients, its absorption, distribution, metabolism, and excretion (pharmacokinetics), as well as its effectiveness in shielding renal function. Committed to enlisting individuals diagnosed with ANCA-associated vasculitis who are also experiencing Rapidly Progressive Glomerulonephritis, the study's cohorts are set to begin.
This medical condition is notorious for causing a swift and severe decline in renal performance, leading to the necessity for interventions such as dialysis or organ transplantation. Although existing treatment options exist, they mainly focus on managing the condition rather than preventing the acute renal deterioration that unfolds prior to disease management.
Dr. Luigi Manenti, in his capacity as Alentis's Chief Medical Officer, expressed excitement over the innovative strides lixudebart might represent in tackling ANCA-RPGN. He noted that this is not lixudebart's first foray into clinical trials, with an earlier study having targeted advanced liver fibrosis. Dr. Manenti emphasized that this current study paves the way for lixudebart's application in other renal diseases marked by significant CLDN1 expression, like lupus nephritis, IgA nephropathy, and diabetic nephropathy.
Professor Per Ivarsen from Aarhus University Hospital in Denmark, serving as the lead investigator for the trial, shared his enthusiasm upon initiating treatment in the first subject. The commencement of the study is seen as a vital step towards evaluating lixudebart's capacity to manage this critical renal condition. He anticipates an informative journey as patient participation increases and study findings emerge.
Professor David Jayne, an expert in Clinical Autoimmunity at Cambridge University, highlighted the significance of the study, pinning hopes on lixudebart as a potential solution to restore renal function in patients afflicted by severe nephritis consequent to ANCA-associated vasculitis. Citing the prevalence of chronic and terminal renal conditions within this patient set, he underscored the importance of the study to shed light on the initial safety and effectiveness of lixudebart for these patients.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
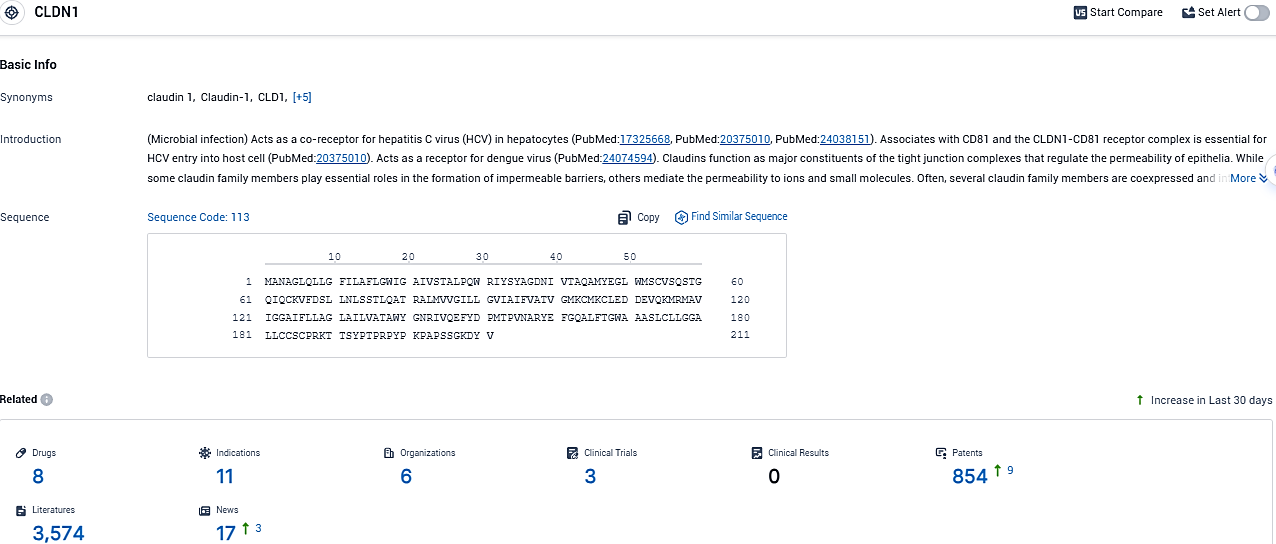
According to the data provided by the Synapse Database, As of December 12, 2023, there are 8 investigational drugs for the CLDN1 target, including 11 indications, 6 R&D institutions involved, with related clinical trials reaching 3, and as many as 854 patents.
Lixudebart, formerly named ALE.F02, is a first-in-class monoclonal antibody developed for liver, lung and kidney fibrosis. It specifically targets a unique CLDN1 epitope exposed in fibrotic tissue to stop progression and even reverse disease. Lixudebart is an investigational antibody that was well tolerated and without any serious safety concerns in a Phase 1 single- and multiple-ascending dose study in healthy volunteers.