CSL & Arcturus' sa-mRNA Technology May Provide Longer Immunity than Standard COVID Boosters
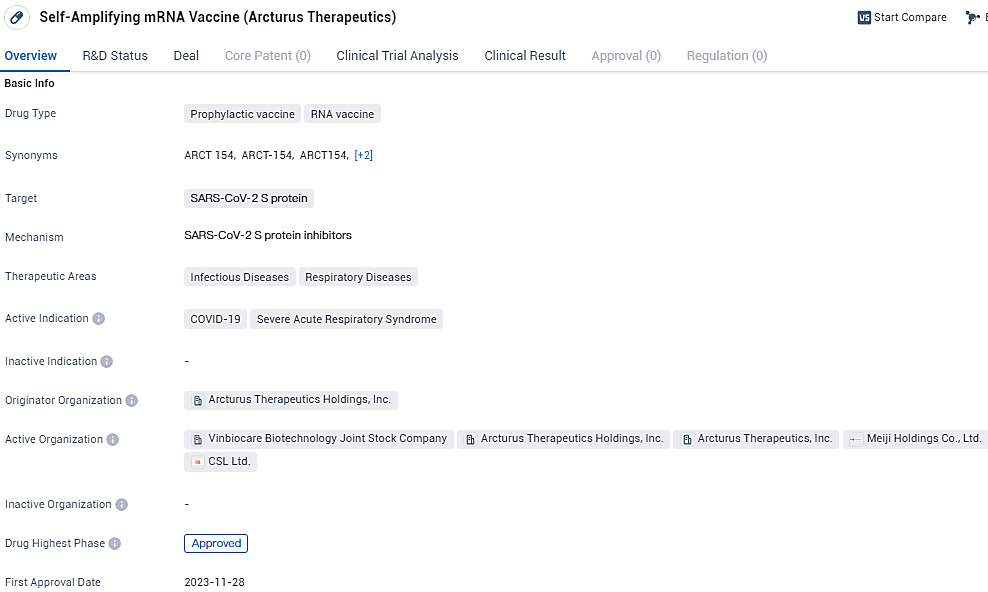
International biotech front-runner CSL in collaboration with Arcturus Therapeutics disclosed the outcomes of a supplementary review of a Phase 3 clinical trial. This evaluation was focused on assessing the efficacy of a lower-dosage booster injection of ARCT-154, which holds the distinction of being the inaugural, sanctioned, self-replicating mRNA vaccine for COVID-19. This was measured against a standard dosage of a traditional mRNA vaccine targeting COVID-19. The dose of ARCT-154 used was only a fraction—specifically one-sixth—of the dose utilized for the vaccine known as Comirnaty.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Six months after vaccination, new findings suggest that ARCT-154 elicits a more sustained immune reaction compared to Comirnaty, with both the initial Wuhan version and the Omicron BA.4/5 strain demonstrating longer-lasting antibody levels.
Jonathan Edelman, M.D., Senior Vice President at the Vaccines Innovation Unit of CSL, highlighted the study's implications. "These findings lend further credence to the unique capacity of sa-mRNA to offer extended COVID-19 protection at reduced dosage levels," he remarked. "Our foremost commitment is to safeguard the populace against viral respiratory illnesses, and we are eager to accumulate and disseminate findings at the one-year post-booster interval."
Pad Chivukula, Ph.D., Arcturus Therapeutics' Chief Scientific Officer, connected these outcomes with earlier Phase 3 studies and recent regulatory green light in Japan. "Together, these insights reinforce the promise of our cutting-edge vaccine approach, showcasing not just sustained immunity at conservative dosages but also potential advantages when juxtaposed with traditional mRNA vaccines," he commented.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
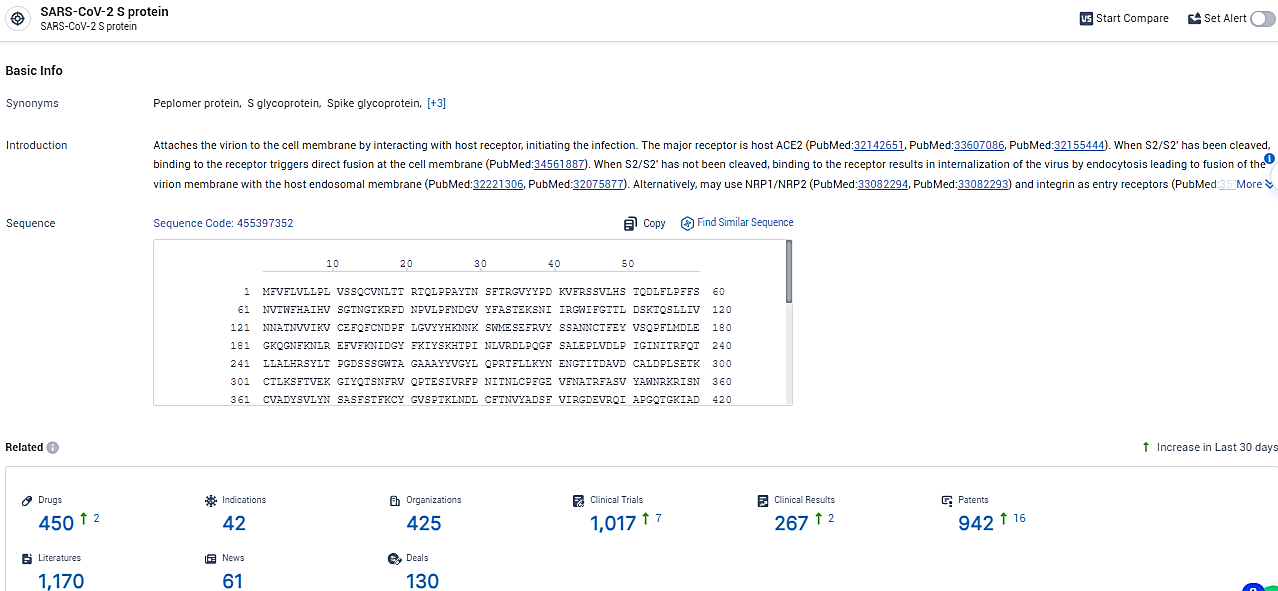
According to the data provided by the Synapse Database, As of February 18, 2024, there are 450 investigational drugs for the SARS-CoV-2 S protein target, including 42 indications, 425 R&D institutions involved, with related clinical trials reaching 1017, and as many as 942 patents.
ARCT-154 developed by Arcturus Therapeutics is a prophylactic RNA vaccine that targets the SARS-CoV-2 S protein. It is intended for the prevention of COVID-19 and other respiratory diseases. The drug has reached the highest phase of development, indicating approval, and is expected to receive its first global approval in November 2023, with Japan being the first country to grant approval.