Incyte Secures Sole Worldwide Rights for Development and Sales of the Drug Tafasitamab (Monjuvi®)
Incyte has declared the establishment of an asset acquisition deal with MorphoSys AG, granting Incyte unique worldwide privileges for the drug tafasitamab. This therapeutic agent, designed to target CD19 with an Fc-enhanced humanized formulation, is commercially known within the United States as Monjuvi® (tafasitamab-cxix) and is branded as Minjuvi® (tafasitamab) in international markets.
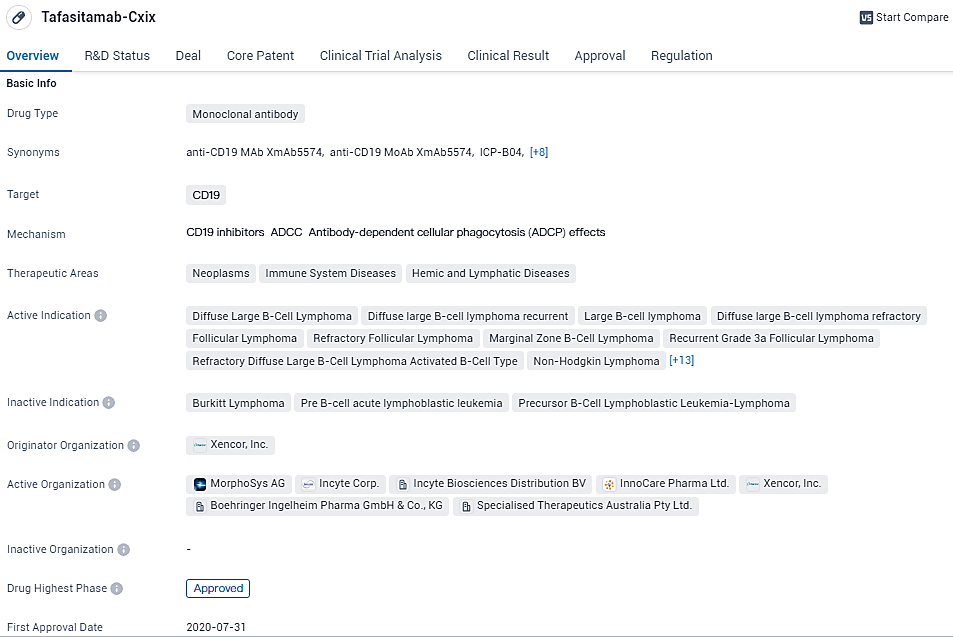
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In a recently formed pact with MorphoSys, Incyte has secured exclusive international privileges for tafasitamab, seizing comprehensive oversight for its advancement and market release. This grants Incyte the opportunity to enhance operational effectiveness and achieve synergy savings in expenditures.
"The acquisition of exclusive worldwide rights to tafasitamab along with complete authority over its progression and marketing processes promises substantial improvements in our operational activities and synergy in costs," commented Hervé Hoppenot, the Chief Executive Officer of Incyte.
Under the initial partnership, MorphoSys and Incyte jointly managed the financial aspects and were involved together in the clinical progression and marketing strategies of tafasitamab within the United States, while Incyte maintained privileged rights in territories beyond the U.S. As per the revised agreement terms, Incyte will compensate MorphoSys with a payment of $25 million, acquiring exclusive global rights for the development and marketing of tafasitamab.
Moving forward, Incyte is set to independently document both revenue and costs pertaining to the U.S. market activities for tafasitamab. MorphoSys will no longer partake in the potential to receive subsequent milestone payments, profit-sharing, or royalty compensations. The terms of this arrangement are being implemented with immediate effect.
Tafasitamab, presently approved for certain uses, is also under assessment in pivotal trials. It is being considered as a potential treatment in first-line therapy for Diffuse Large B Cell Lymphoma (DLBCL), and for patients with either relapsed or refractory follicular lymphoma and relapsed or refractory marginal zone lymphoma.
As a monoclonal antibody, tafasitamab targets CD19 and is designed as an immunotherapy treatment. It employs a humanized Fc-modified structure. In 2010, the exclusive worldwide rights to tafasitamab were licensed to MorphoSys by Xencor, Inc. Featuring an XmAb engineered Fc region, tafasitamab activates the destruction of B-cells by inducing apoptosis and engaging immune effector functions such as ADCC (Antibody-Dependent Cell-Mediated Cytotoxicity) and ADCP (Antibody-Dependent Cellular Phagocytosis).
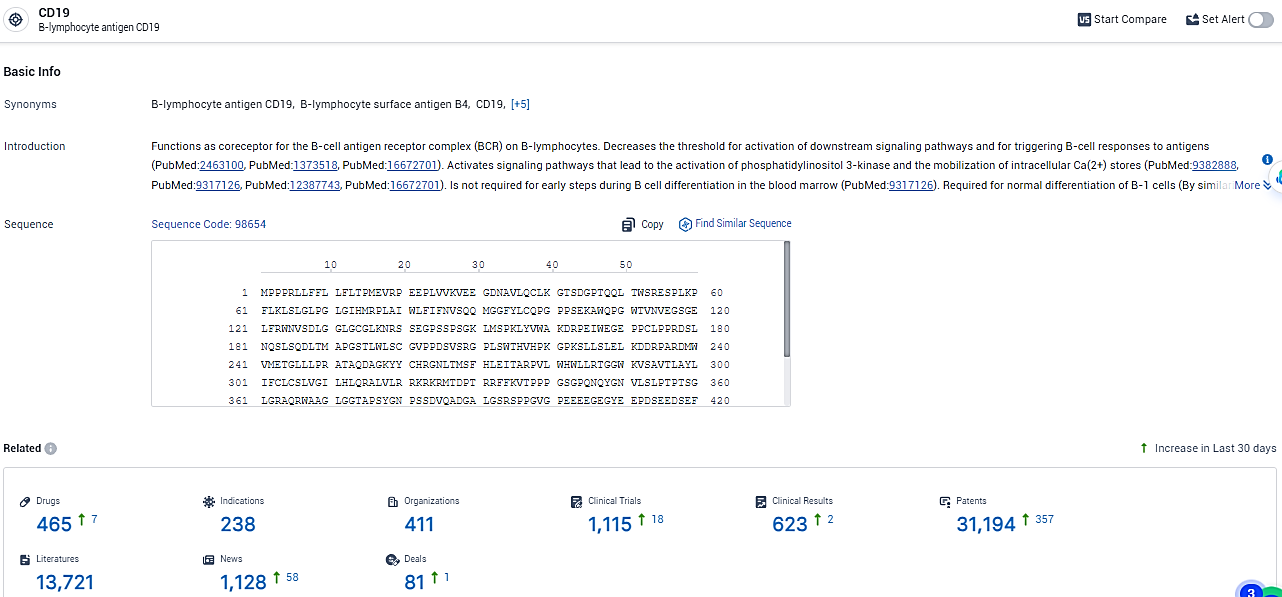
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 18, 2024, there are 465 investigational drugs for the CD19 target, including 238 indications, 411 R&D institutions involved, with related clinical trials reaching 1115, and as many as 31194 patents.
In Europe, tafasitamab received conditional marketing authorization in combination with lenalidomide, followed by Minjuvi monotherapy, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are not eligible for autologous stem cell transplant. Tafasitamab is being clinically investigated as a therapeutic option in B-cell malignancies in several ongoing combination trials. Its safety and efficacy for these investigational uses have not been established in pivotal trials.