CSPC Pharmaceutical Group's Claudin6/CD137 Dual Antibody NBL-028 has been approved for clinical trials, aimed at treating late-stage solid tumors
Recently, CSPC Pharmaceutical announced that its independently developed bispecific antibody targeting Claudin6/CD137, NBL-028, has received approval from the National Medical Products Administration (NMPA) in China for clinical trial applications. The indications include but are not limited to late-stage tumors expressing Claudin6 such as testicular cancer, ovarian cancer, non-small cell lung cancer, and endometrial cancer.
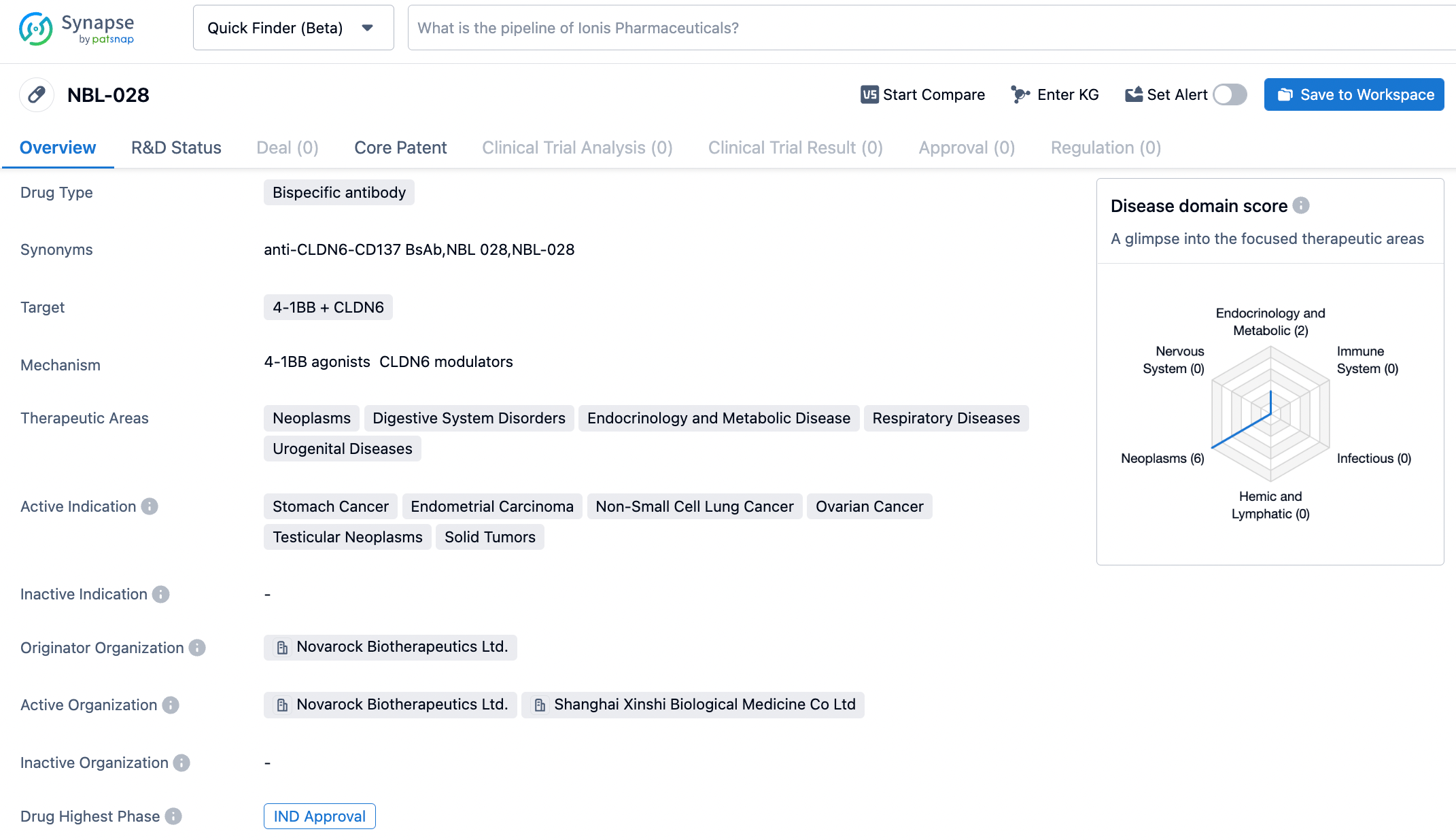
NBL-028 is a bispecific antibody developed by CSPC Pharmaceutical targeting full human-derived Claudin6 and CD137. Upon binding with Claudin6 on the surface of tumor cells in the tumor microenvironment (TME), it can selectively activate the CD137 co-stimulatory pathway in T cells and other immune cells, thereby selectively killing tumor cells. Preclinical studies have demonstrated good efficacy and safety of NBL-028. Claudin 6 is a tight junction protein in the Claudin family, expressed at high levels in various human malignant tumors, but almost not or not expressed in normal tissues. CD137 (or 4-1BB) is an induced co-stimulatory receptor, is a member of the Tumor Necrosis Factor Receptor (TNFR) superfamily, and plays a key role in T cell proliferation, survival, cytotoxic activity, memory formation, and the regulation of other immune cell functions. Preclinical studies have shown that NBL-028 demonstrates excellent efficacy and safety.
At the 2022 AACR Annual Meeting, NovaRock presented this bispecific antibody. The abstract showed that NBL-028 can bind to Claudin 6 with high affinity and specificity, activates CD137 in a CLDN6-dependent manner, and has characteristics of rapid on and off CD137 binding. NBL-028 showed strong CLDN6-dependent T cell activation and T cell-mediated cytotoxicity in vitro. In mouse models, NBL-028 generated strong antitumor effects and immune memory, with no detectable liver damage or systemic toxicity. Tumor infiltrating lymphocyte (TIL) analysis also indicated that NBL-028 treatment can induce CD45+ immune cell infiltration, reduce the Treg/CD8 ratio, and increase the M1/M2 macrophage ratio. In addition, NBL-028 is designed with a proprietary molecular scaffold, demonstrating excellent developability in terms of stability, yield, and ease of purification.
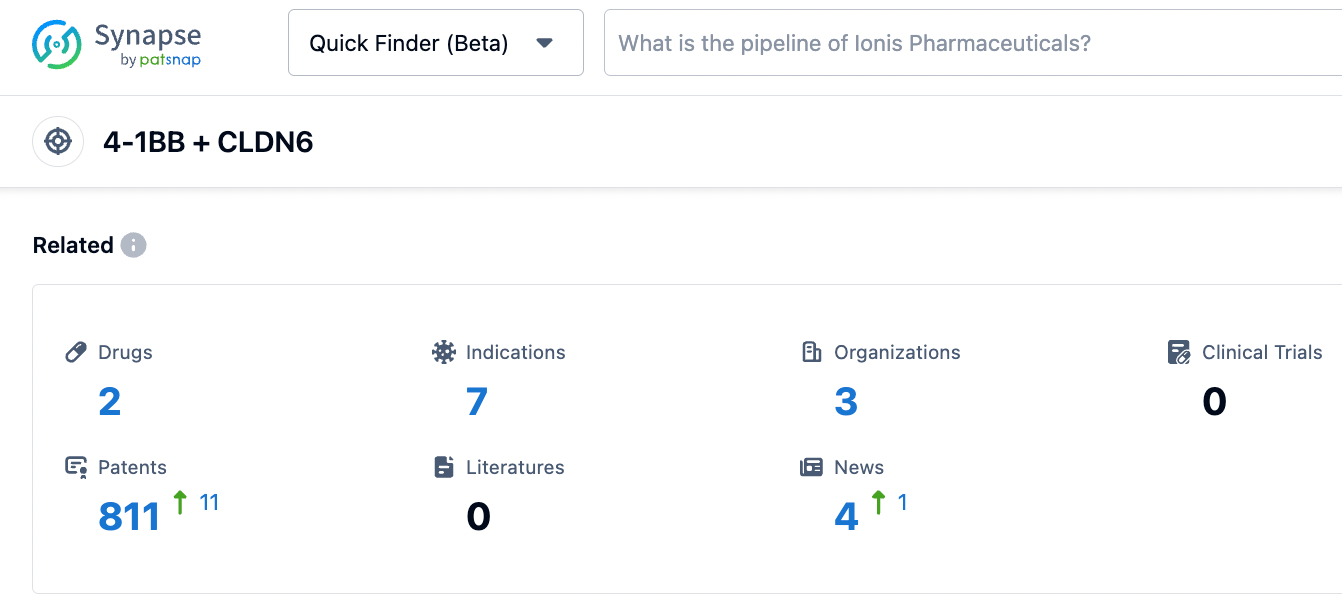
According to information revealed by the synapse database, as of October 27, 2023, there are 2 drugs in research for Claudin6/CD137 target, with 7 indications, researched by 3 organizations, and having as many as 810 patents...... With the entry of NBL-028 into clinical trials, once successful, it is expected to open up incremental space. We look forward to CSPC Pharmaceutical's subsequent performance in bispecific antibodies, ADCs, and other new antibody drugs.