CStone Pharmaceuticals releases Phase 3 GEMSTONE-303 clinical trial data of anti-PD-L1 monoclonal antibody sugemalimab, for the treatment of gastric cancer
At the 2023 European Society for Medical Oncology (ESMO) conference, a multi-center, randomized, placebo-controlled Phase III registration clinical trial GEMSTONE-303 of CStone Pharmaceuticals' anti-PD-L1 monoclonal antibody, sugemalimab, was selected for a late-breaking abstract (LBA) report. According to the conference announcement on the ESMO official website, this is the first reported Phase III trial of an anti-PD-L1 monoclonal antibody for this disease, and the study achieved a dual primary endpoint of progression-free survival (PFS) and overall survival (OS).
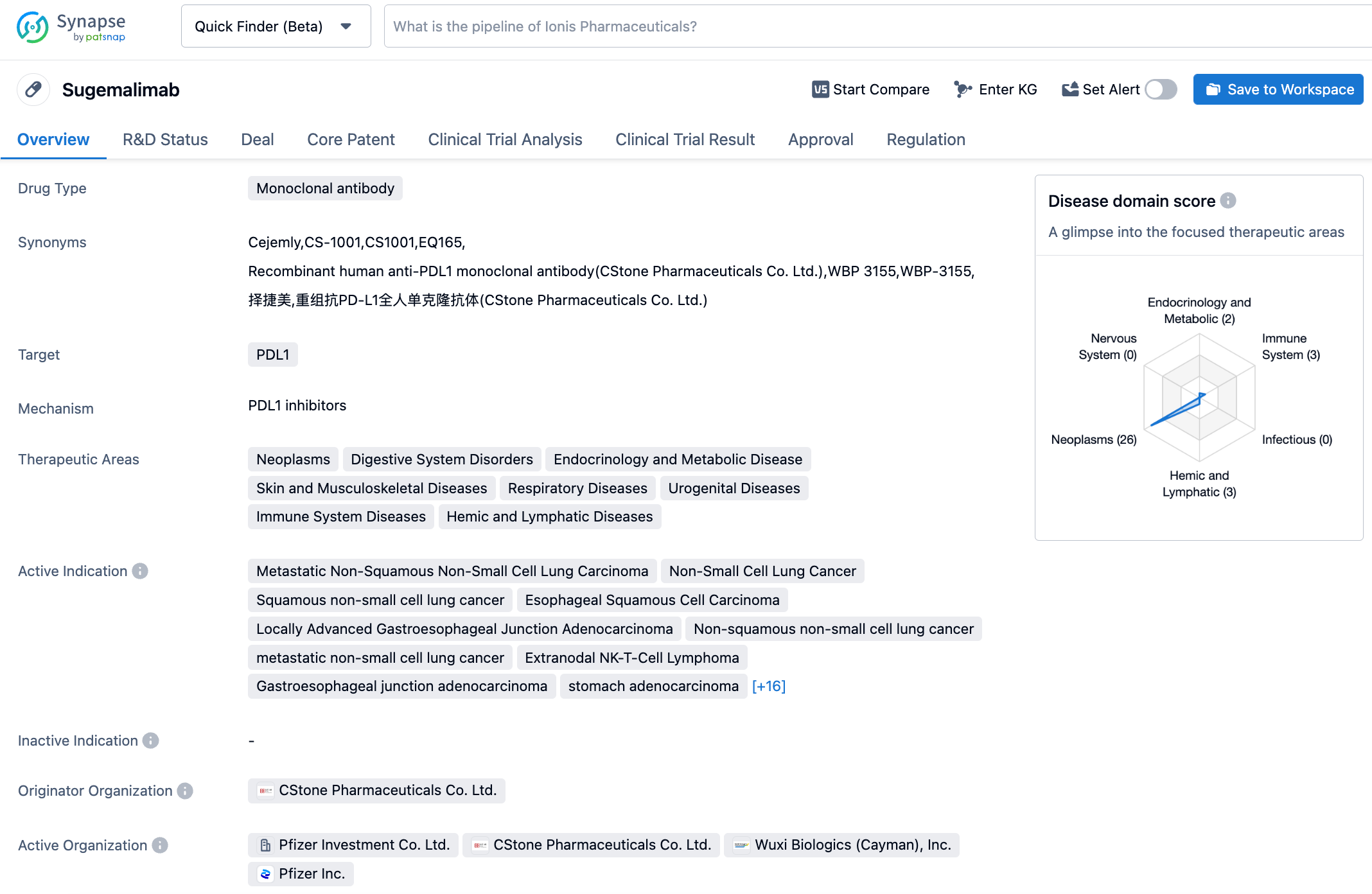
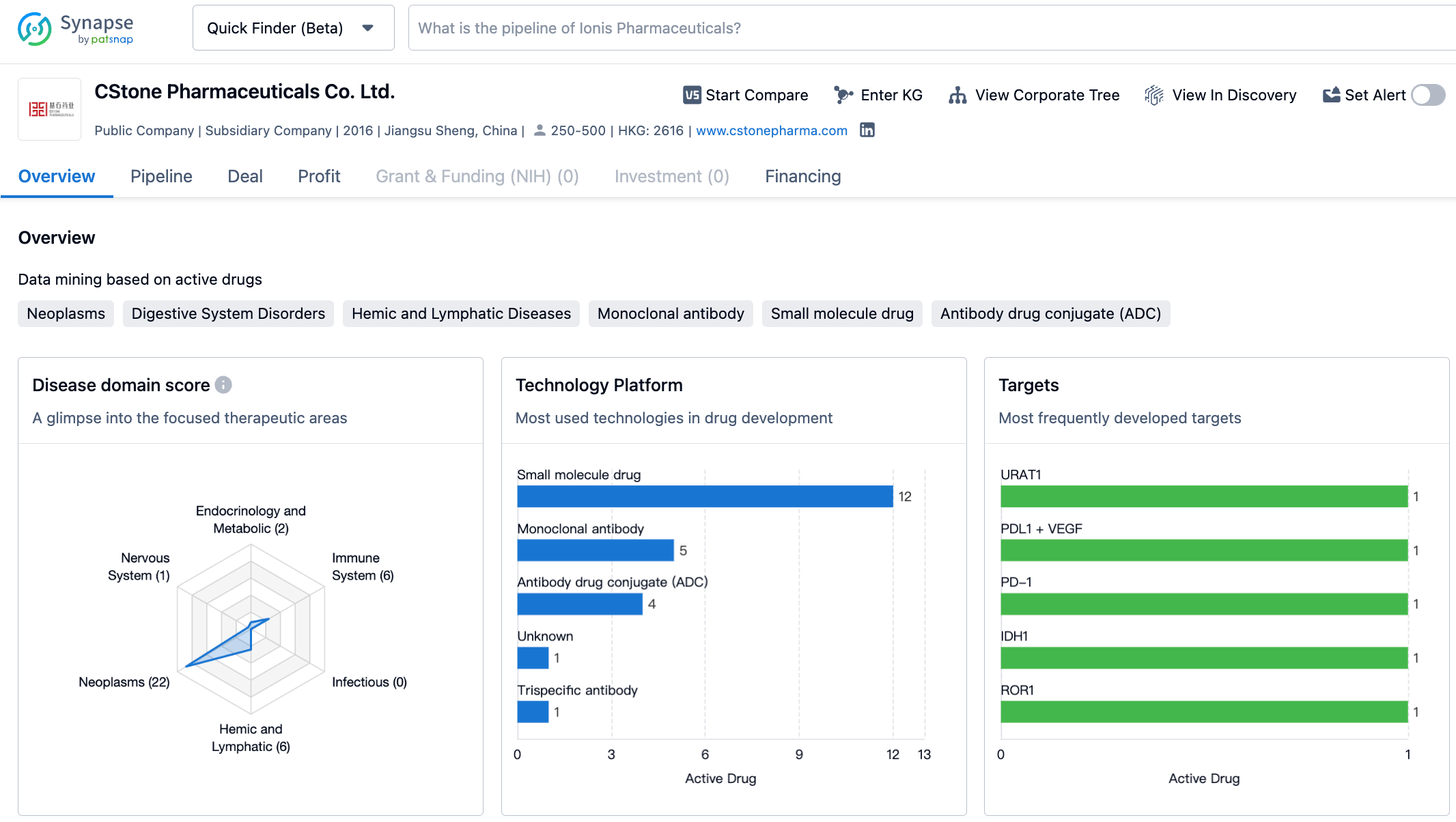
Sugemalimab is an anti-PD-L1 monoclonal antibody developed by CStone Pharmaceuticals. Currently, sugemalimab has been approved for two indications in China: in combination with Pemetrexed and Carboplatin for first-line treatment of EGFR gene mutation-negative and ALK-negative metastatic non-squamous non-small cell lung cancer, and in combination with paclitaxel and Carboplatin for first-line treatment of metastatic squamous non-small cell lung cancer (December 2021); and for consolidated treatment of inoperable stage III non-small cell lung cancer (NSCLC) patients after simultaneous or sequential radiochemotherapy without disease progression (May 2022). Notably, sugemalimab has been included in the "2022 CSCO Guidelines for the Diagnosis and Treatment of Non-Small Cell Lung Cancer" and is recommended for first-line treatment of stage IV non-squamous/squamous NSCLC patients and for consolidated treatment of stage III NSCLC patients after concurrent or sequential radiochemotherapy. In February 2023, the NMPA accepted a new indication for sugemalimab in combination with chemotherapy for the first-line treatment of inoperable locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma, which is currently under review.
GEMSTONE-303 is a multi-center, randomized, placebo-controlled Phase III registration clinical trial aimed at evaluating the efficacy and safety of sugemalimab in combination with the CAPOX chemotherapy regimen (Oxaliplatin + Capecitabine) as the first-line treatment for inoperable locally advanced or metastatic gastric adenocarcinoma or gastroesophageal junction adenocarcinoma with PD-L1 expression ≥5%. The primary endpoints of this study are PFS and OS assessed by investigators, while secondary endpoints include PFS assessed by a blinded Independent Central Review (BICR) and objective response rate (ORR) and duration of response (DoR) evaluated by investigators. In November 2022, the GEMSTONE-303 study achieved the primary endpoint of PFS and showed a significant benefit trend in the interim analysis of OS. Compared with the placebo combined with chemotherapy control group, the combination of sugemalimab and chemotherapy significantly improved the PFS assessed by investigators, and the difference has statistical and clinical significance.
According to the information disclosed in the synapse database as of October 21, 2023, there are a total of 437 drugs under research for the PD-L1 target, with 226 potential indications, further being studied by 392 institutions, involving 3004 related clinical trials, and as many as 36685 patents. In October 2020, CStone Pharmaceuticals authorized EQRx to develop and commercialize sugemalimab and anti-PD-1 monoclonal antibody CS1003 outside of Greater China, with the collaboration involving up to $1.3 billion. EQRx has submitted applications to the Medicines and Healthcare Products Regulatory Agency (MHRA) and the European Medicines Agency (EMA) for the first-line treatment of metastatic NSCLC with sugemalimab in combination with chemotherapy, hoping for successful market entry of sugemalimab in the international market.