Decoding trihexyphenidyl hydrochloride: A Comprehensive Study of its R&D Trends
Trihexyphenidyl hydrochloride's R&D Progress
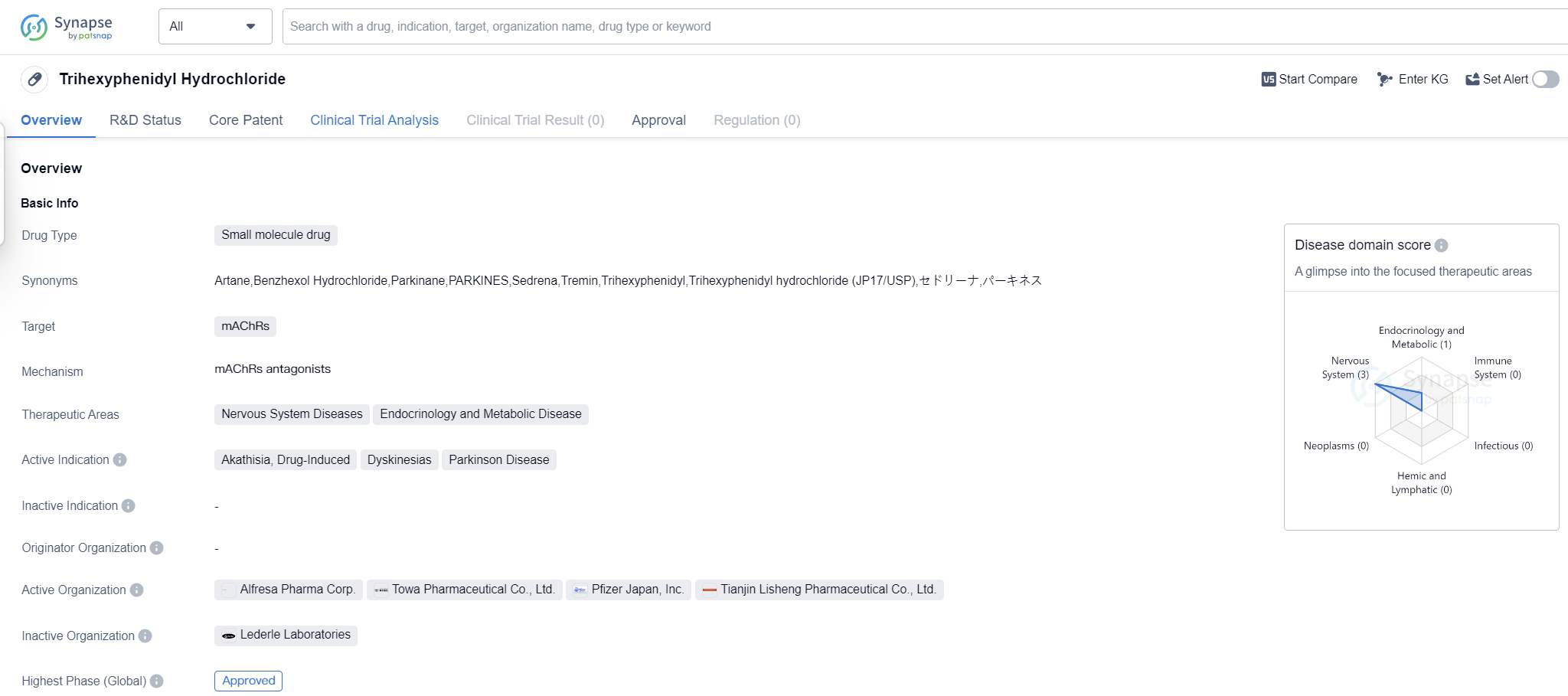
Trihexyphenidyl Hydrochloride is a small molecule drug that primarily targets muscarinic acetylcholine receptors (mAChRs). It is used in the treatment of various nervous system diseases and endocrinology and metabolic diseases. The drug has been approved for the treatment of akathisia, drug-induced dyskinesias, and Parkinson's disease.
Trihexyphenidyl Hydrochloride was first approved in the United States in May 1949, making it one of the oldest drugs in the pharmaceutical market. It has since gained global approval and is widely used in the medical field. The drug's mechanism of action involves blocking the action of acetylcholine, a neurotransmitter in the brain, which helps alleviate the symptoms associated with the aforementioned conditions.
Akathisia is a condition characterized by restlessness and an inability to sit still, often caused by the use of certain medications. Trihexyphenidyl Hydrochloride has been found to be effective in reducing these symptoms, providing relief to patients. Similarly, drug-induced dyskinesias, which are involuntary movements caused by certain medications, can also be managed with the use of this drug.
Parkinson's disease, a neurodegenerative disorder, is another condition for which Trihexyphenidyl Hydrochloride is approved. It helps alleviate the motor symptoms associated with the disease, such as tremors, stiffness, and difficulty with movement. By targeting mAChRs, the drug helps restore the balance of neurotransmitters in the brain, improving motor function in Parkinson's patients.
Trihexyphenidyl Hydrochloride has reached the highest phase of development which is approved globally. Being approved for several indications demonstrates its versatility in treating various conditions related to the nervous system. Its long history of use since 1949 further supports its reliability and effectiveness.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for trihexyphenidyl hydrochloride: mAChRs antagonists
mAChRs antagonists refers to muscarinic acetylcholine receptor antagonists. Muscarinic acetylcholine receptors (mAChRs) are a type of receptor found in the central and peripheral nervous systems that bind to the neurotransmitter acetylcholine. Antagonists are substances that bind to a receptor without activating it, thereby blocking the receptor's normal function.
In the context of biomedicine, mAChRs antagonists are a class of drugs that specifically inhibit the activity of muscarinic acetylcholine receptors. These drugs are used for various therapeutic purposes, such as treating overactive bladder, reducing excessive salivation, managing symptoms of Parkinson's disease, and mitigating side effects of anesthesia. By blocking the mAChRs, these antagonists can help regulate the balance of acetylcholine signaling in the body and alleviate certain conditions or symptoms.
Drug Target R&D Trends for trihexyphenidyl hydrochloride
Muscarinic acetylcholine receptors (mAChRs) are a class of G-protein coupled receptors found in various tissues throughout the human body. They play a crucial role in mediating the effects of acetylcholine, a neurotransmitter involved in numerous physiological processes. mAChRs are involved in regulating heart rate, smooth muscle contraction, glandular secretion, and neuronal signaling in the central and peripheral nervous systems. Dysfunction of mAChRs has been implicated in various diseases, including Alzheimer's disease, Parkinson's disease, and asthma. Understanding the role of mAChRs is essential for developing targeted therapies that modulate their activity, potentially leading to improved treatments for these conditions.
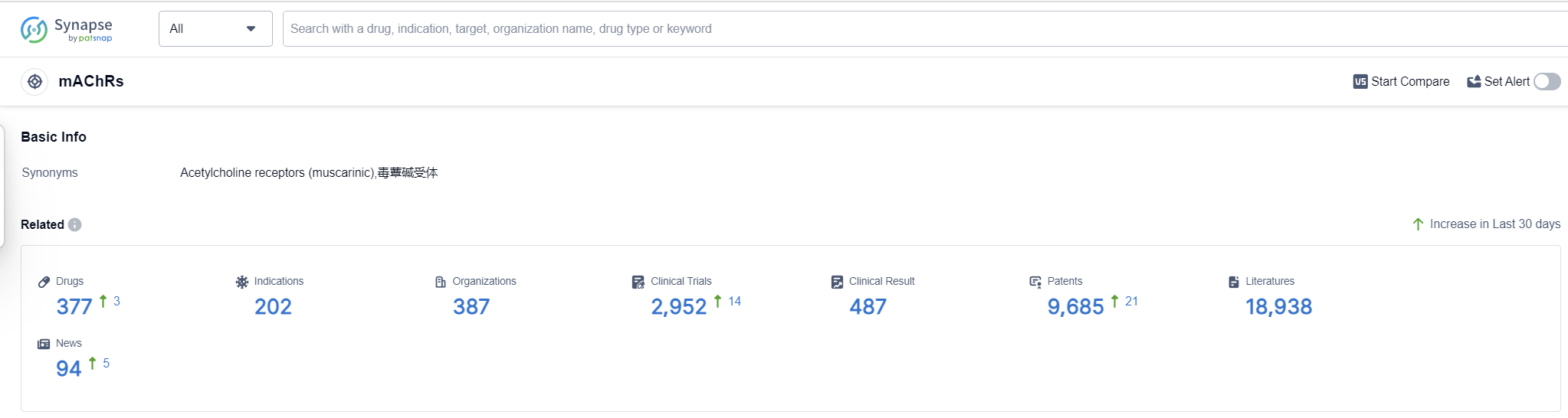
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 377 mAChRs drugs worldwide, from 387 organizations, covering 202 indications, and conducting 2952 clinical trials.
Based on the analysis of the target mAChRs, Pfizer Inc. and C.H. Boehringer Sohn AG & Co. KG are the companies growing fastest under this target. The highest stage of development is reached by Pfizer Inc. with 8 approved drugs. Indications such as Pulmonary Disease, Chronic Obstructive and Urinary Bladder, Overactive have the highest number of approved drugs. Small molecule drugs are progressing rapidly, indicating intense competition. China is developing rapidly under the target mAChRs, with the highest number of approved drugs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Trihexyphenidyl Hydrochloride is a small molecule drug that targets mAChRs and is approved for the treatment of akathisia, drug-induced dyskinesias, and Parkinson's disease. Its first approval dates back to 1949 in the United States, and it has since gained global approval. The drug's mechanism of action involves blocking acetylcholine, providing relief to patients suffering from these conditions. Its widespread use and long history of approval highlight its efficacy and safety in the field of biomedicine.