Cytoki Pharma Begins Phase 2 Trial of CK-0045 for Obesity and Type 2 Diabetes
Cytoki Pharma, ApS (Cytoki), a biotechnology firm in the clinical development phase, is at the forefront of developing a novel category of therapeutics that utilize IL-22 biology to enhance outcomes for metabolic disorders. The company has announced that the initial patient has been administered CK-0045 in a Phase 2 proof-of-concept study to assess its efficacy, safety, and tolerability in patients diagnosed with obesity and type 2 diabetes.
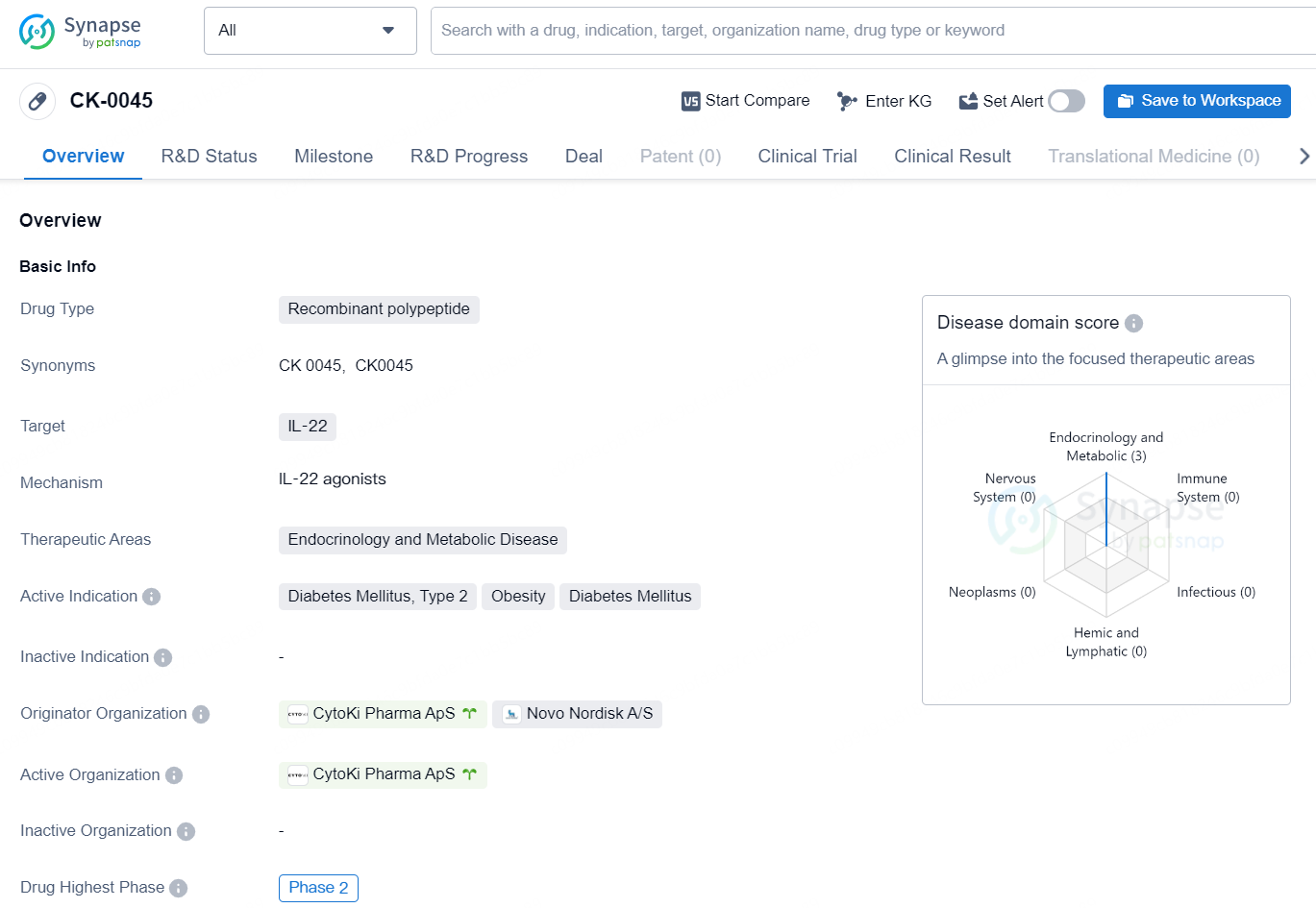
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Obesity and diabetes rank as significant public health concerns, affecting over one billion people globally. The introduction of incretin-based therapies has brought new treatment possibilities; however, there is still a need for innovative strategies that provide cardiometabolic advantages beyond mere weight loss," stated Anne Louise Kjølbye, Ph.D., MBA, Chief Development Officer at Cytoki. "CK-0045 represents a fresh approach to treating metabolic disorders, potentially extending healthy longevity through weight loss that preserves muscle mass while offering additional health benefits."
CK-0045 functions as an IL-22 analogue and utilizes established lipidation technology to enhance its circulation time in the body, allowing for subcutaneous administration and presenting first-in-class opportunities for addressing obesity and type 2 diabetes. Data from the Phase 1 clinical trial involving healthy participants confirmed effective target engagement and dose-dependent decreases in body mass, along with improvements in low-density lipoprotein (LDL) cholesterol, blood insulin levels, and insulin resistance. These findings, paired with a favorable safety assessment, endorse further clinical development of CK-0045.
“The promising results from the Phase 1 trial of CK-0045 highlight the validity of its distinct IL-22-based mechanism as a prospective impactful treatment for obesity and type 2 diabetes,” commented Dr. Carel le Roux, a Professor of Experimental Pathology at University College Dublin. “We look forward to Cytoki's advancements as it continues to assess CK-0045 in clinical settings and works on its broader IL-22-derived program to create innovative treatments for metabolic disorders.”
A 16-week randomized, double-blind, placebo-controlled Phase 2 trial (NCT06611930) will assess the efficacy, safety, and tolerability of two dosages of CK-0045, given subcutaneously on a weekly basis, in 90 participants suffering from obesity and type 2 diabetes. The study will investigate a variety of metabolic benefits associated with IL-22 modulation, including weight loss, modifications in HbA1c, insulin sensitivity metrics, and lipid profiles. Results are expected in the early part of 2026.
Furthermore, Cytoki is actively progressing a comprehensive portfolio of IL-22-based programs aimed at addressing significant unmet needs in both metabolic disorders and inflammatory bowel disease.
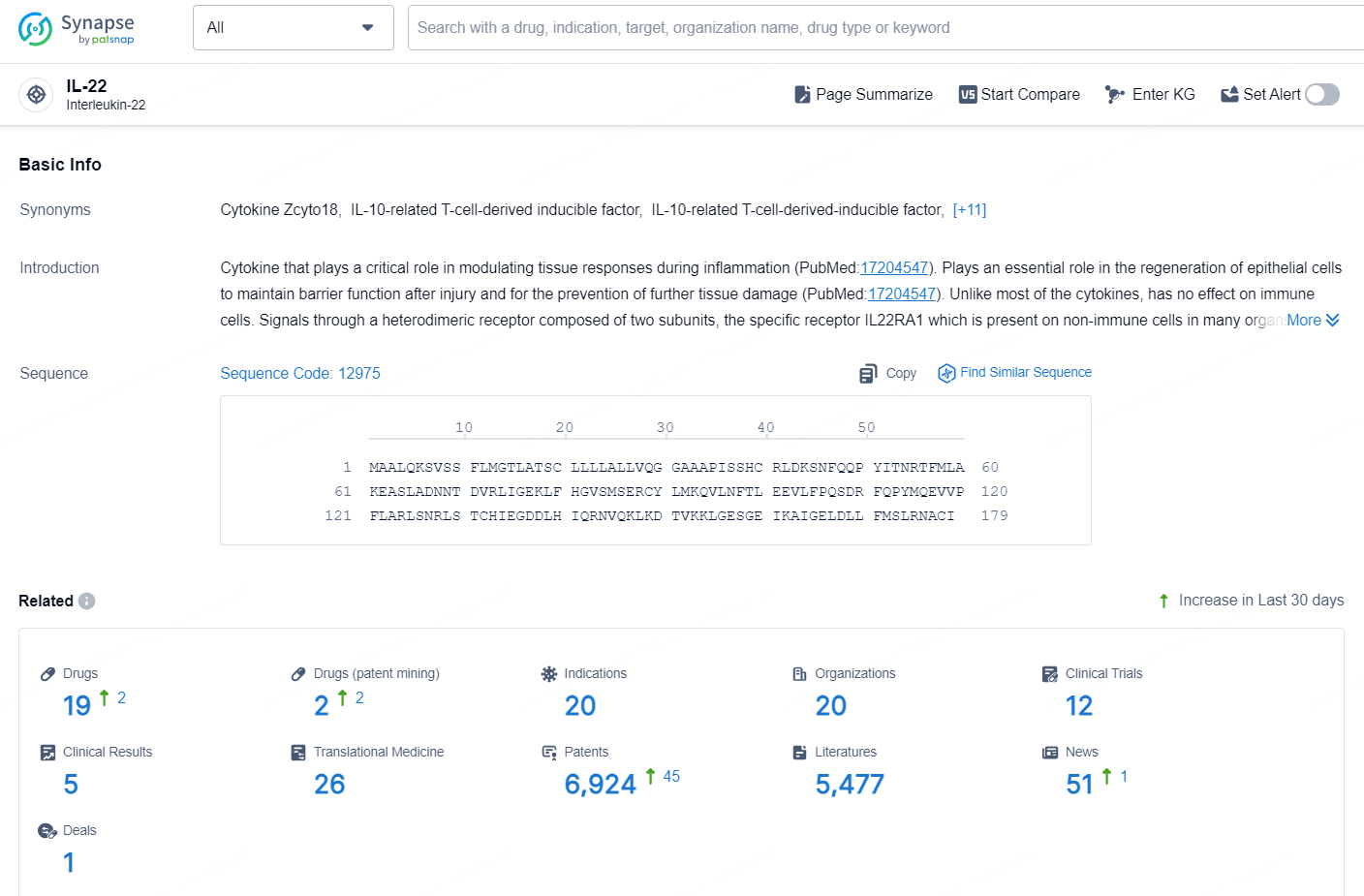
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 5, 2024, there are 19 investigational drugs for the IL-22 target, including 20 indications, 20 R&D institutions involved, with related clinical trial reaching 12, and as many as 6924 patents.
CK-0045 is a recombinant polypeptide drug that targets IL-22 and is being developed for the therapeutic areas of endocrinology and metabolic disease. Its active indications include diabetes mellitus, type 2, obesity, and diabetes mellitus. The drug is being developed by CytoKi Pharma ApS and Novo Nordisk A/S.