FDA Accepts Ionis's New Drug Application for HAE Preventive Treatment, Donidalorsen
Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) has announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for donidalorsen. This investigational medication is designed to target RNA for the preventive treatment of hereditary angioedema (HAE) attacks in both adults and children aged 12 years and older. The FDA plans to take action by August 21, 2025, in accordance with the Prescription Drug User Fee Act (PDUFA). The submission to the FDA is supported by favorable findings from both monthly and bi-monthly administration in the pivotal Phase 3 OASIS-HAE and OASISplus (open-label extension and switch) studies, in addition to data from the ongoing Phase 2 open-label extension study.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
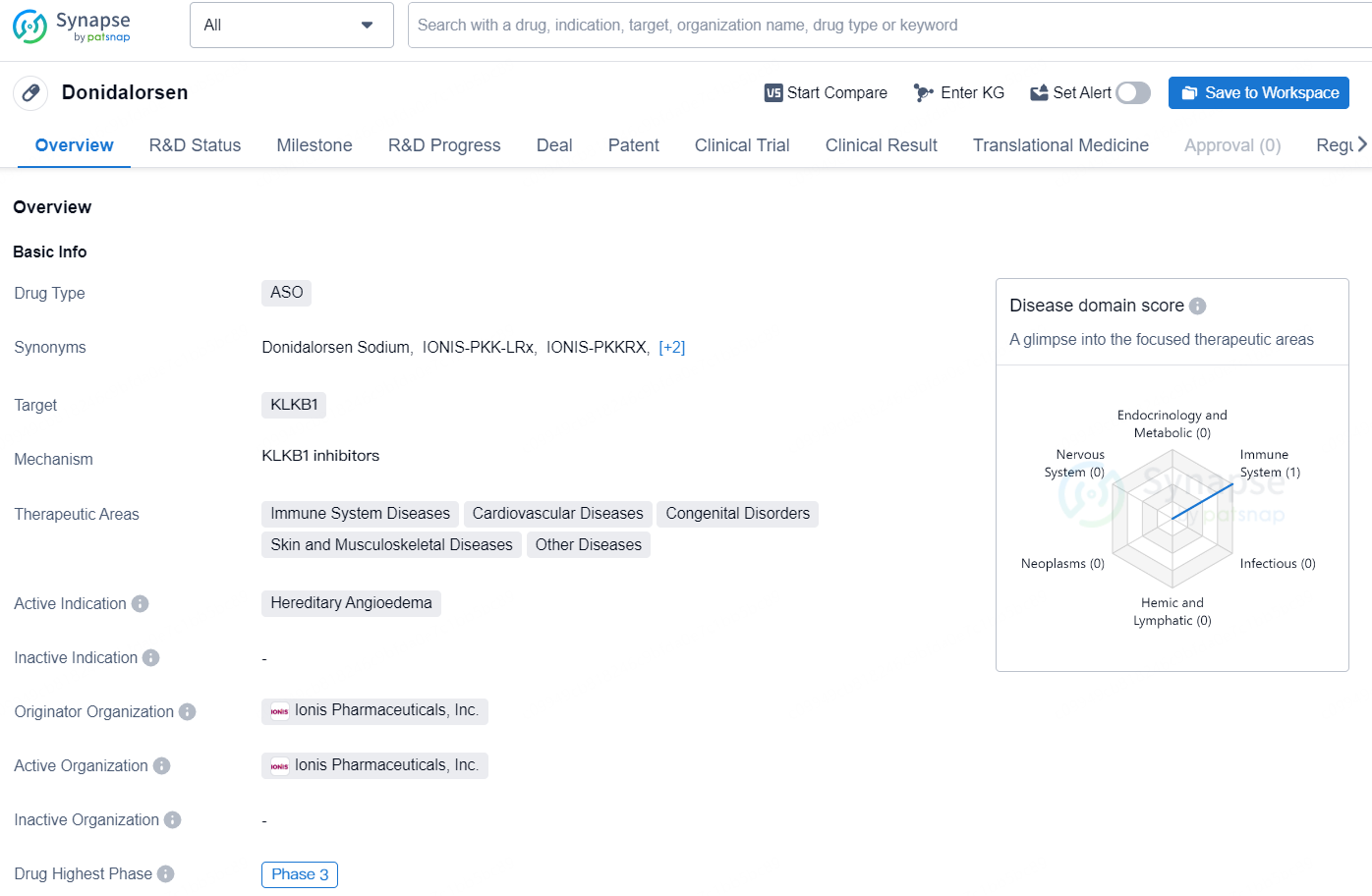
Hereditary Angioedema (HAE) is a rare genetic disorder that can pose significant health risks, characterized by recurrent instances of severe swelling (angioedema) affecting various parts of the body, such as the hands, feet, genitals, abdomen, face, and/or throat. Donidalorsen is formulated to decrease the synthesis of prekallikrein (PKK), thereby disrupting the cascade that triggers HAE episodes.
“Even with existing therapies, many individuals with HAE still suffer from painful and potentially hazardous breakthrough attacks. Drawing from the comprehensive clinical data gathered from the Phase 3 OASIS-HAE and OASISplus trials, along with the recent three-year outcomes from our Phase 2 OLE study, we are confident that donidalorsen can enhance the preventive treatment framework for those affected by HAE,” stated Brett Monia, Ph.D., CEO of Ionis.
“With the FDA's acceptance of our donidalorsen New Drug Application (NDA), we are on track for our second independent product launch next year, pending approval, which aligns with our commitment to consistently provide new medications for patients facing severe health conditions.” The FDA earlier granted donidalorsen Orphan Drug Designation in 2023. Otsuka, which holds exclusive rights to market donidalorsen in Europe and the Asia-Pacific region, is set to submit a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) this year.
Ionis recently shared new findings from the Phase 3 and Phase 2 OLE studies at the 2024 American College of Allergy, Asthma & Immunology (ACAAI) Annual Scientific Meeting, indicating that donidalorsen produced significant and durable reductions in HAE attacks, with an overall sustained mean reduction in attack rates of 96% from baseline maintained over a span of three years in the ongoing Phase 2 OLE study. Across all three trials, donidalorsen was well-tolerated, exhibiting no serious treatment-emergent adverse events (TEAEs) directly linked to the drug. Most adverse events (AEs) reported were mild or moderate in nature, with injection site reactions being the most frequently observed AE.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
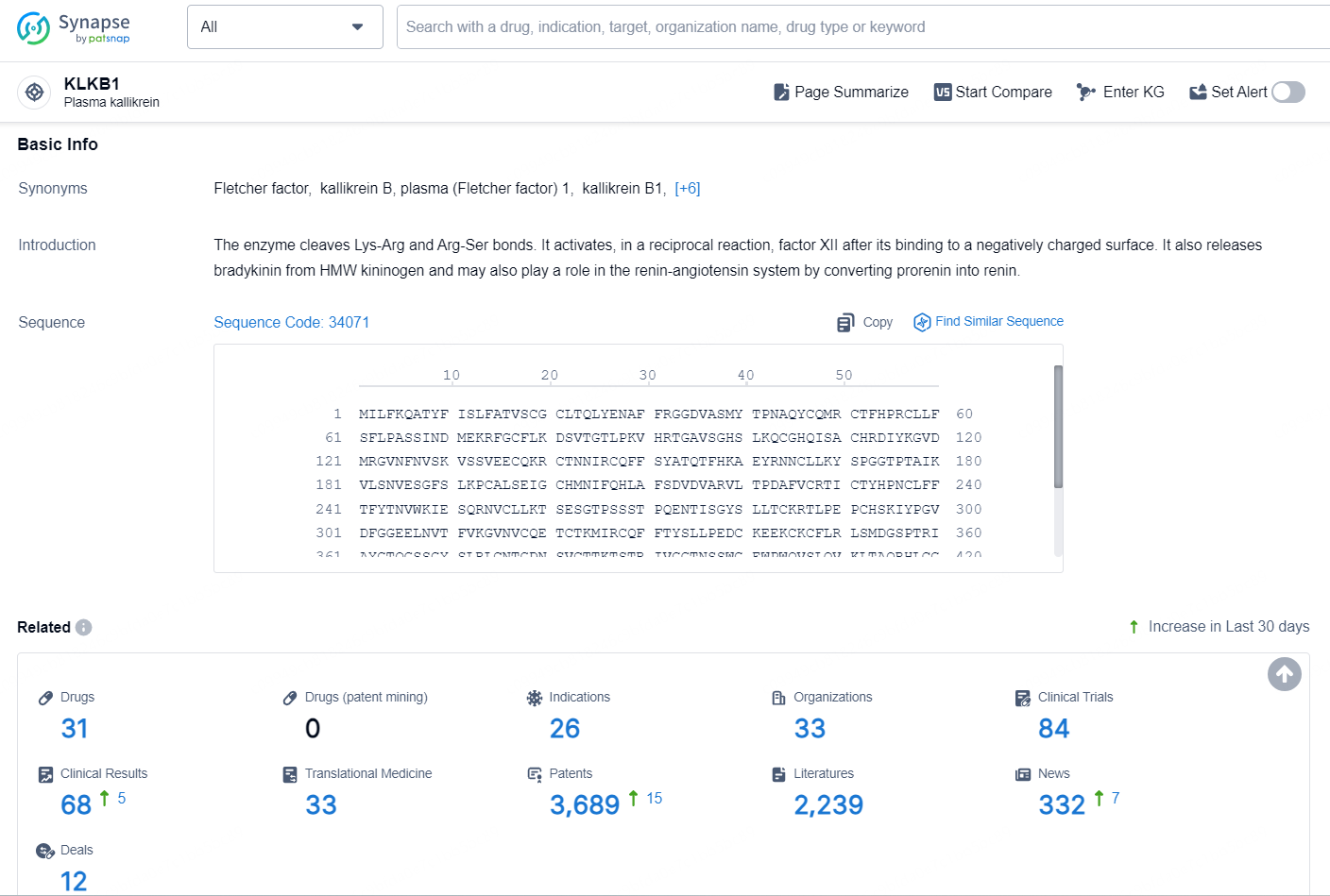
According to the data provided by the Synapse Chemical, As of November 5, 2024, there are 31 investigational drugs for the KLKB1 target, including 26 indications, 33 R&D institutions involved, with related clinical trial reaching 84, and as many as 3689 patents.
Donidalorsen is an antisense oligonucleotide (ASO) drug that targets the KLKB1 gene. It is being developed for the treatment of various therapeutic areas, including immune system diseases, cardiovascular diseases, congenital disorders, skin and musculoskeletal diseases, as well as other diseases. The active indication for Donidalorsen is hereditary angioedema, a rare genetic disorder characterized by recurrent episodes of severe swelling in various parts of the body.