Cytoki Pharma Reports Promising Phase 1 Results Indicating CK-0045 Could Enhance Cardiometabolic Health
Cytoki Pharma, ApS, a biotechnology company in the clinical development phase and at the forefront of creating a new class of drugs leveraging IL-22 biology for better metabolic disease outcomes, has reported favorable data from a Phase 1 trial. This study assessed the safety, tolerability, and pharmacokinetics of their leading lipidated IL-22 candidate, CK-0045, in both healthy individuals and those with obesity.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
As the landscape for treating obesity progresses, there is still a necessity for strategies that induce sustained disease modification through healthy weight reduction and extensive weight loss-independent metabolic effects,” stated Rasmus Jorgensen, Ph.D., Founder and CEO of Cytoki Pharma. “We think CK-0045 presents a unique chance to tackle obesity and type 2 diabetes, either alone or in conjunction with other clinical methods, and we are heartened by this initial data supporting CK-0045’s novel mechanism of action.”
IL-22 is a non-immunomodulatory cytokine specifically targeting epithelial tissues like the gut and liver. Previously published preclinical findings indicate lipidated IL-22’s potential to decrease body weight and enhance glucose regulation in mice via a new action mechanism.
Outcomes from the Phase 1 trial show successful transfer of preclinical results to humans, with confirmed target engagement based on biomarkers derived from the liver and gut. Pharmacokinetic data affirm the feasibility of weekly subcutaneous dosing. CK-0045 led to dose-dependent reductions in body mass, along with beneficial decreases in low-density lipoprotein cholesterol, blood insulin levels, and insulin resistance—especially in participants with diminished insulin sensitivity.
CK-0045 was found to be safe and tolerable in the multiple ascending dose segment of the study, with all but one participant completing the dosing. The most frequently observed side effects were mild skin reactions, and there was a notable lack of significant gastrointestinal effects.
The available clinical data support moving CK-0045 into Phase 2 proof-of-concept studies in individuals with obesity and type 2 diabetes to further evaluate the clinical relevance of the effects observed. The initiation of Phase 2 is expected in the latter half of 2024.
CK-0045 is a long-lasting analogue of interleukin-22 (IL-22), an unusual, non-immunomodulatory cytokine specifically targeting epithelial cells. Licensed from Novo Nordisk A/S, CK-0045 uses validated technology to enhance the pharmacologic properties of the endogenous IL-22 protein, aiming to create a unique, first-in-class therapy with the potential to address a wide array of metabolic diseases, including obesity and type 2 diabetes, and conditions marked by epithelial damage, such as inflammatory bowel disease.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
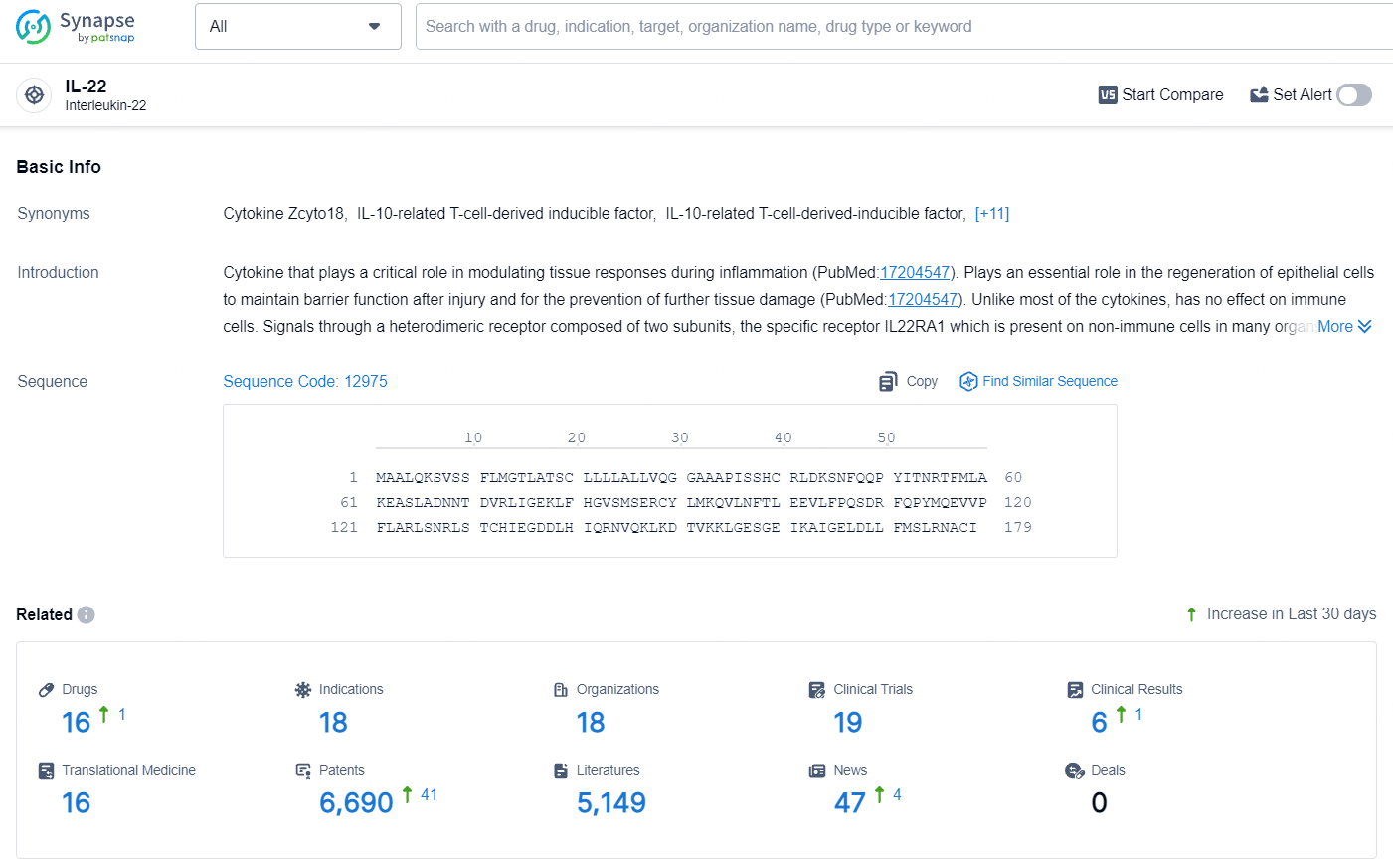
According to the data provided by the Synapse Database, As of July 24, 2024, there are 16 investigational drugs for the IL-22 target, including 18 indications, 18 R&D institutions involved, with related clinical trials reaching 19, and as many as 6690 patents.
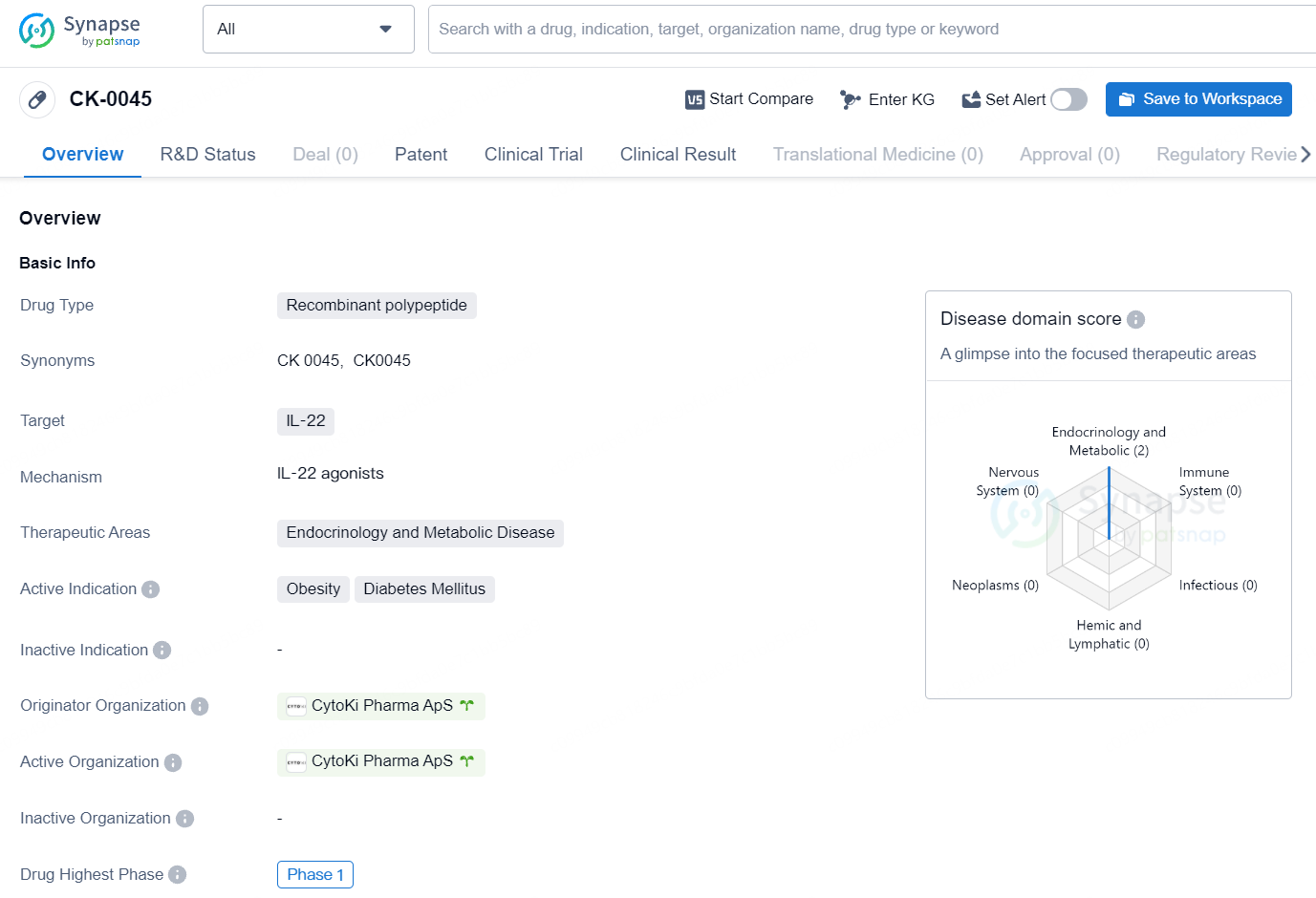
CK-0045 primary target is IL-22, and it is intended for use in the therapeutic areas of endocrinology and metabolic disease. The active indications for CK-0045 include obesity and diabetes mellitus. At the time of reporting, CK-0045 has reached the highest global phase of Phase 1 in its development. No further details on the drug's specific mechanism of action, clinical trial data, or potential market release date are available at this time.