Is Auvelity approved by the FDA?
Auvelity, under its generic name bupropion and dextromethorphan, has been approved by the FDA for the treatment of major depressive disorder. It is available in the form of an oral tablet, specifically an extended-release formulation containing 105 mg of bupropion and 45 mg of dextromethorphan. This combination falls under the drug class of miscellaneous antidepressants.
What is Bupropion and Dextromethorphan?
Bupropion and dextromethorphan is prescribed for the treatment of major depressive disorder in adults. Bupropion is an antidepressant that works by restoring the balance of certain natural chemicals (neurotransmitters) in the brain, while dextromethorphan is a cough suppressant that also affects the brain chemicals involved in mood and behavior regulation.
Side Effects
Common side effects of bupropion and dextromethorphan include dizziness, headache, diarrhea, drowsiness, dry mouth, excessive sweating, and sexual problems. Serious side effects may include seizures, changes in vision, paranoia, hallucinations, and serotonin syndrome, which is characterized by agitation, hallucinations, fever, fast heart rate, muscle stiffness, twitching, loss of coordination, nausea, and diarrhea.
Warnings and Precautions
Before taking Auvelity (bupropion and dextromethorphan), inform your healthcare provider if you have a history of seizures, eating disorders, alcohol use, or if you've used MAO inhibitors in the past 14 days. Use of MAO inhibitors with Auvelity can lead to dangerous drug interactions. It's also important to monitor for any new or worsening symptoms of depression, anxiety, or suicidal thoughts.
Dosage and Administration
Auvelity (bupropion and dextromethorphan) is typically taken once daily, with the initial dose followed by a maintenance dose administered twice daily, spaced at least 8 hours apart. It should be swallowed whole and not crushed, chewed, or broken. Regular monitoring of blood pressure is advised during treatment.
Interaction with Other Medications
Auvelity (bupropion and dextromethorphan) may interact with other medications, including other antidepressants, drugs for anxiety or mood disorders, theophylline, steroids, insulin or oral diabetes medications, appetite suppressants, smoking cessation aids, and certain street drugs. Always inform your doctor about all medications you are taking, including over-the-counter drugs and supplements.
In conclusion, Auvelity (bupropion and dextromethorphan) is FDA approved for the treatment of major depressive disorder in adults. It combines bupropion, an antidepressant, with dextromethorphan, a cough suppressant, to address mood disorders effectively. As with any medication, it's crucial to follow your doctor's instructions carefully and report any unusual symptoms or side effects promptly.
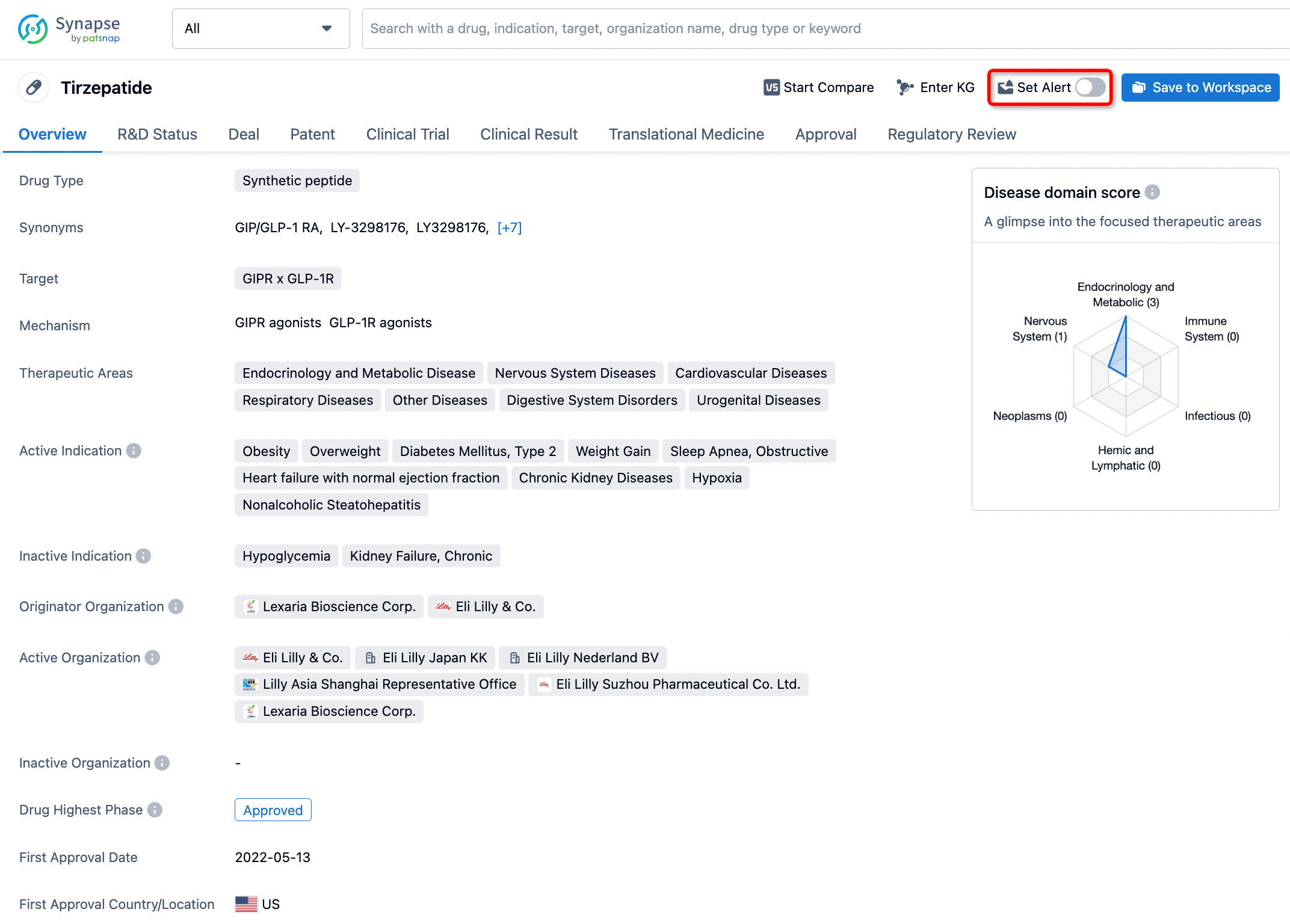
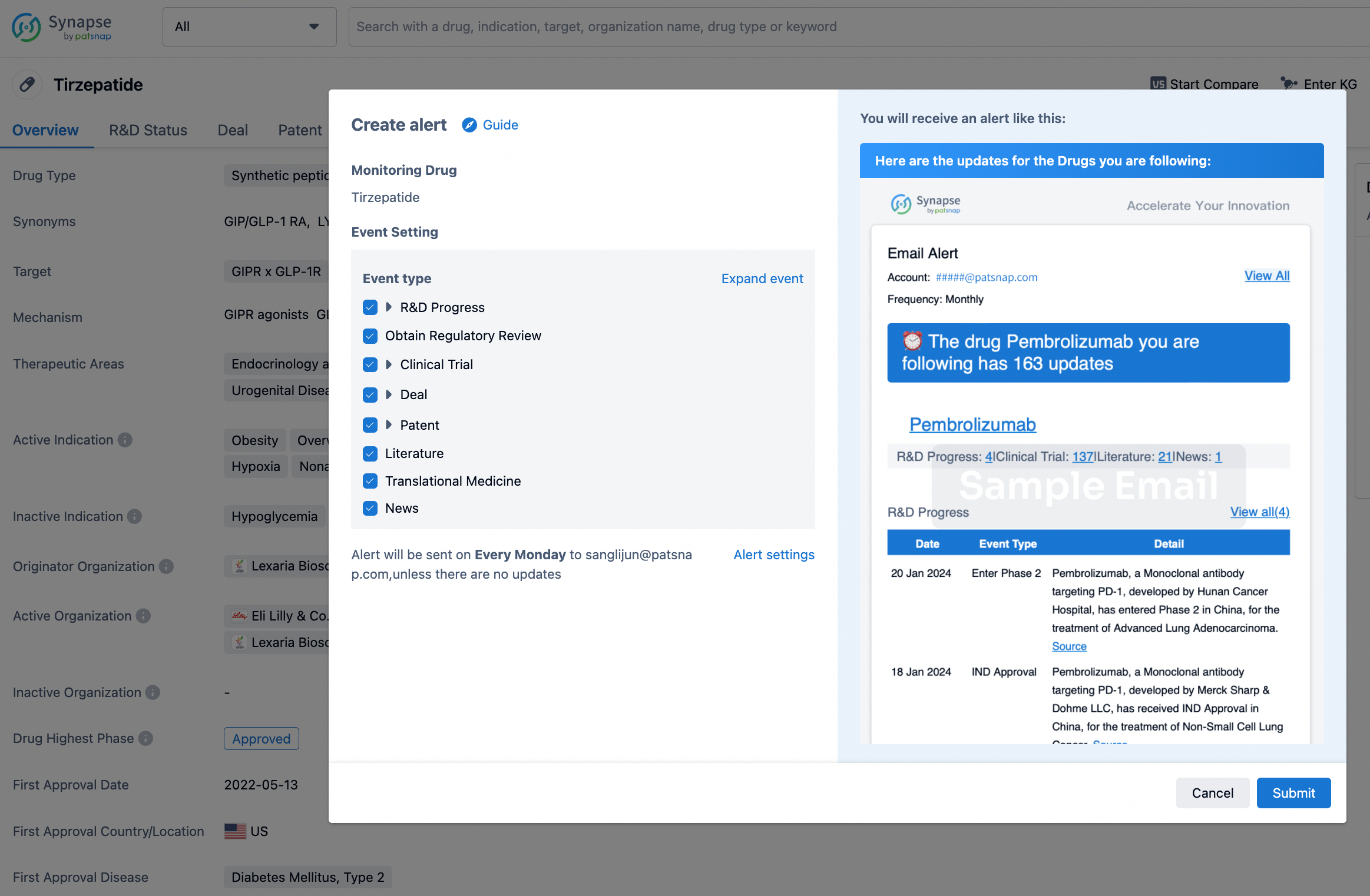
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!