CytomX Therapeutics Announces Promising Phase 1a Results for CX-904 in Dose Escalation Study
CytomX Therapeutics, Inc., a pioneer in conditionally activated and masked biologic therapeutics, has today revealed encouraging early results from the continuing Phase 1a dose escalation trial of CX-904. The data showcased a positive safety profile and verified anti-cancer efficacy. CX-904 is a research-stage, masked, and conditionally activated PROBODY T-cell engager, engineered to aim at the epidermal growth factor receptor (EGFR) present on cancer cells and the CD3 receptor located on T cells in the tumor microenvironment.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"we are excited to announce preliminary findings for CX-904, a cutting-edge masked T-cell engager that represents our commitment at CytomX to developing safer and more effective treatments," remarked Sean McCarthy, D.Phil., CEO and chairman of CytomX.
Sean McCarthy further noted, "The insights gained underscore the culmination of a decade's work at CytomX and hint at vast new applications for T-cell engagers in various cancers and targets. We are eager to delve deeper into the capabilities of this promising compound in various EGFR-positive malignancies, together with charting a more long-range approach in collaboration with our worldwide partner, Amgen."
By the data cutoff on April 16, 2024, the Phase 1 trial had registered 35 patients suffering from advanced metastatic solid cancers commonly recognized for EGFR expression, such as pancreatic, colorectal, non-small cell lung cancer, head and neck squamous cell carcinoma, gastric, and esophageal cancers.
The participants of this study were extensively pre-treated, with a median of four earlier treatments. As of the latest data, 19 individuals were included in the initial non-step dosing groups with dosage targets varying from 0.007 mg to 6 mg. Moreover, 16 participants were later incorporated into step-dosing groups with tocilizumab prophylaxis and dosage targets spanning 5 mg to 10 mg. An ongoing cohort targets a dose of 15 mg.
Up to the recent analysis, CX-904 has exhibited an encouraging safety profile, facilitating patient management on an outpatient basis. Notably, no instances of CRS at any level have been detected in the step-dosing groups. Within the non-step dosing segments, only a single case of Grade 1 CRS was recorded at the highest 6 mg dose.
Early pharmacokinetic and pharmacodynamic assessments align well with the PROBODY TCE action mechanism, showcasing continued masking in blood, along with CD8+ T-cell margination and penetration into the tumor.
The dose escalation and refinement in Phase 1a of CX-904 persist, with ongoing efforts to establish suitable dosages for Phase 2. The Company anticipates a further update on Phase 1a dose escalation by the end year of 2024, which is set to facilitate conversations with our partner, Amgen, on launching Phase 1b expansion cohorts targeting specific EGFR-positive cancer indications.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
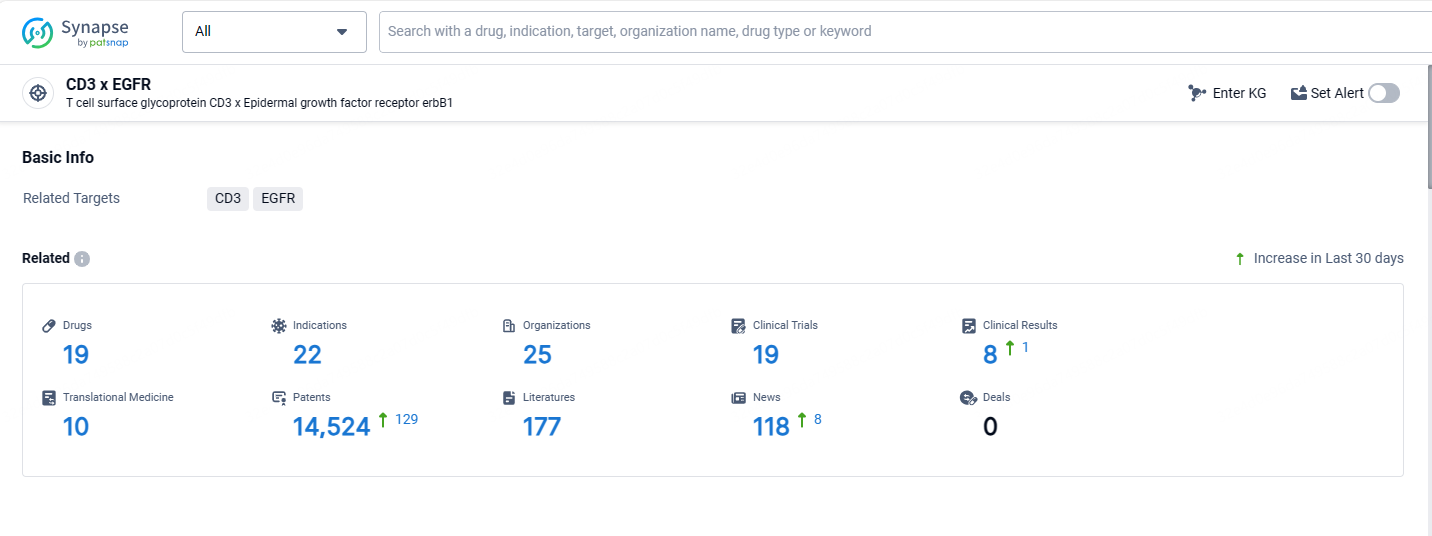
According to the data provided by the Synapse Database, As of May 13, 2024, there are 19 investigational drugs for the CD3 and EGFR targets, including 22 indications, 25 R&D institutions involved, with related clinical trials reaching 19, and as many as 14524 patents.
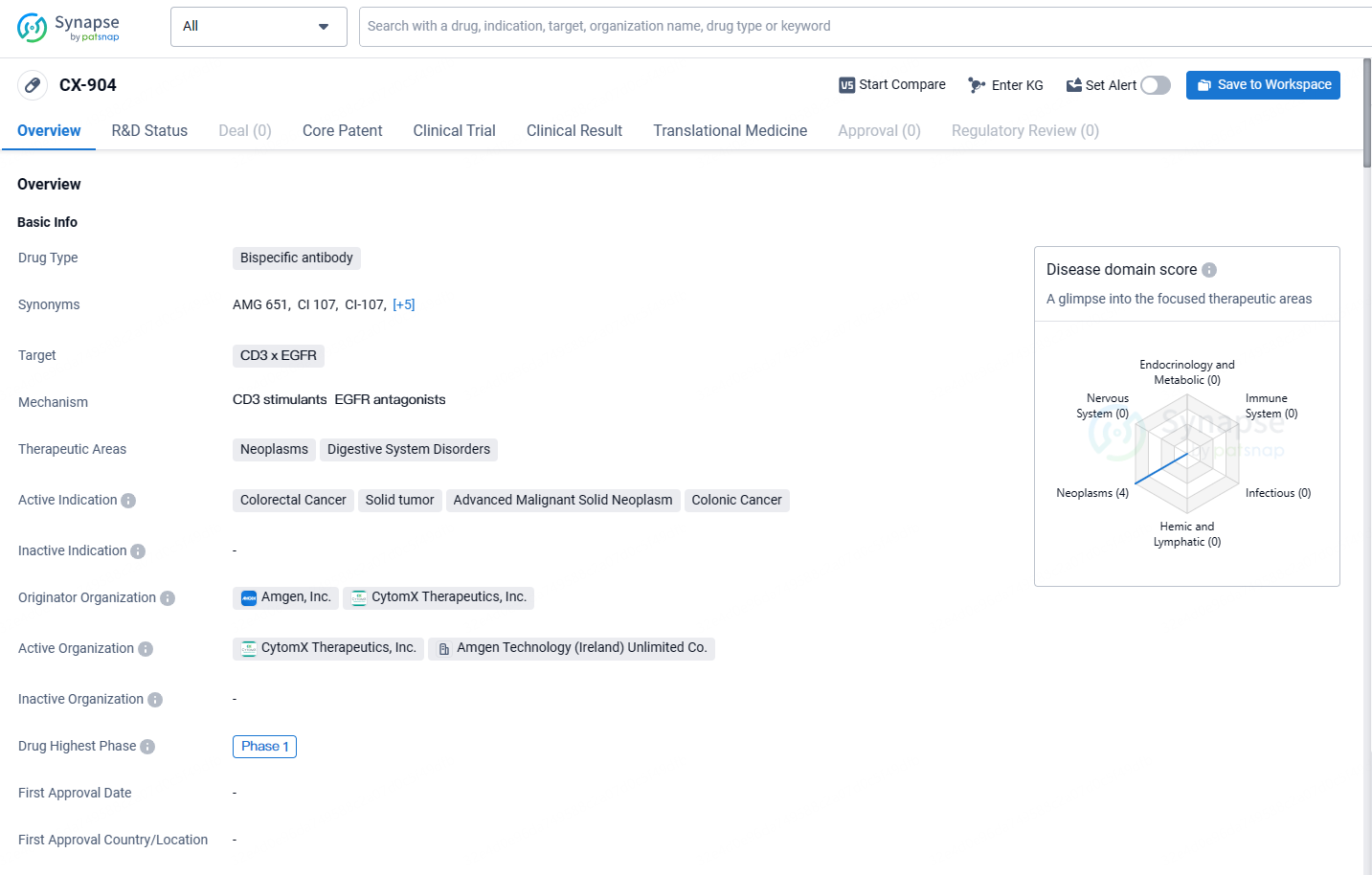
CX-904 targets CD3 x EGFR and is intended for the treatment of colorectal cancer, solid tumors, advanced malignant solid neoplasms, and colonic cancer. The drug is currently in Phase 1, indicating that it is undergoing early-stage clinical trials to assess its safety and efficacy.