Cytonics Finishes Phase 1 Enrollment for CYT-108 Study in Knee Osteoarthritis
Cytonics Corporation, a private biotechnology firm focused on creating biologic treatments for inflammatory musculoskeletal disorders, has revealed that they have completed participant enrollment for their Phase 1 clinical trial of CYT-108. CYT-108 is a recombinant form of the blood serum protease inhibitor alpha-2-macroglobulin, and it is being investigated as a potential disease-modifying therapy for osteoarthritis (OA).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This pioneering clinical trial involving human subjects is a multi-site, 6-month, double-blind randomized controlled trial (RCT) including 22 participants, allocated equally between the treatment and placebo groups. It is designed to assess the safety and effectiveness of CYT-108 in individuals with unilateral, mild-to-moderate primary osteoarthritis (OA) of the knee. The main safety measures will examine CYT-108’s tolerability when administered through an intra-articular injection directly into the affected knees. Secondary endpoints will look at improvements in patients' self-reported pain and joint mobility, while an exploratory endpoint will measure peptide fragments in blood serum as an indicator of cartilage degradation. Thus far, there have been no adverse events related to the drug, and CYT-108 has been well-tolerated by all participants.
"We are ecstatic to have finished enrolling all 22 subjects across our three clinical locations in Australia, exceeding our recruitment timeline by more than a month. This will accelerate our Phase 1 clinical study report and our Investigational New Drug (IND) application submission to the FDA in 2025, moving us closer to providing the first and only disease-modifying treatment for osteoarthritis. We are extremely impressed by the recruitment capabilities of our clinical sites and eagerly anticipate continuing our collaboration with these researchers and clinicians as we progress into Phase 2," said Joey Bose, President & CEO of Cytonics Corp.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
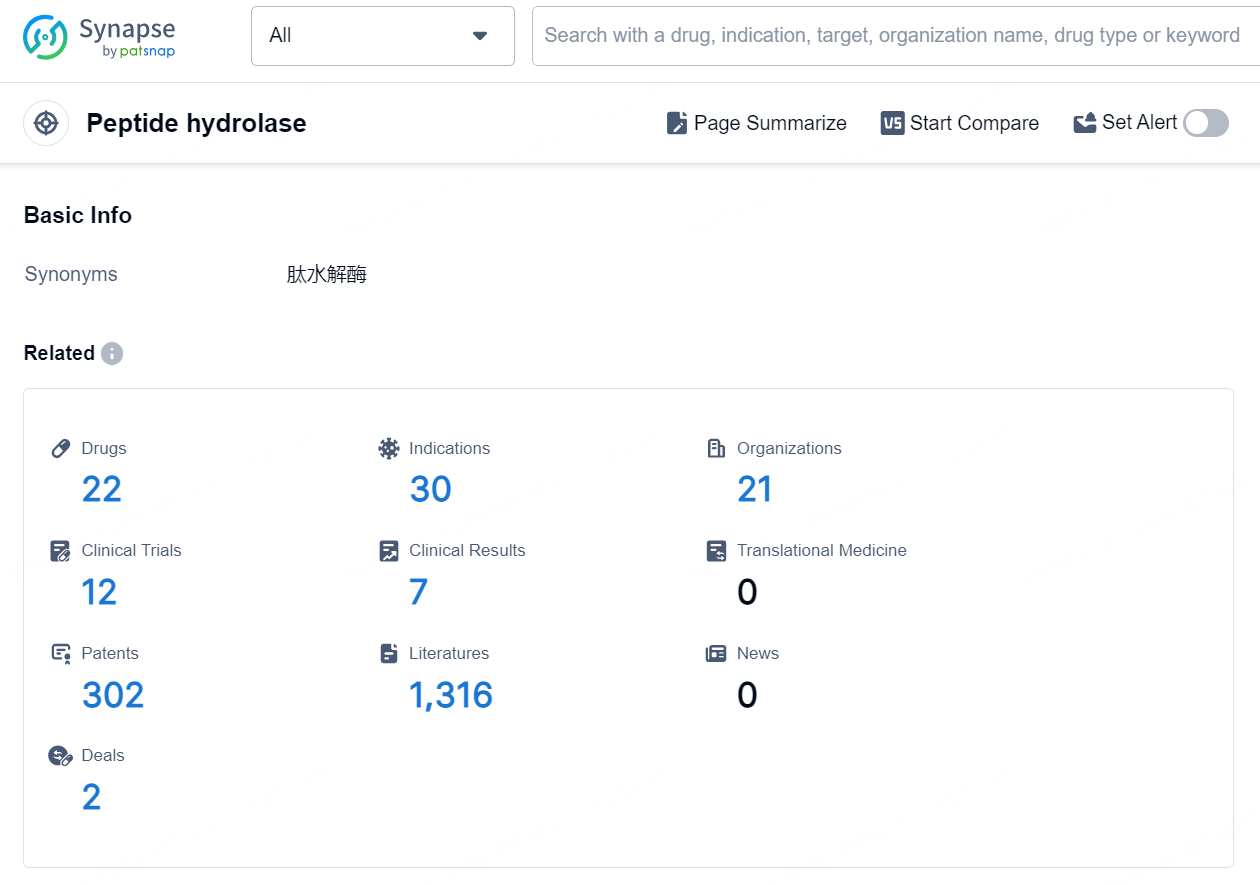
According to the data provided by the Synapse Database, As of September 9, 2024, there are 22 investigational drugs for the Peptide hydrolase targets, including 30 indications, 21 R&D institutions involved, with related clinical trials reaching 12, and as many as 302 patents.
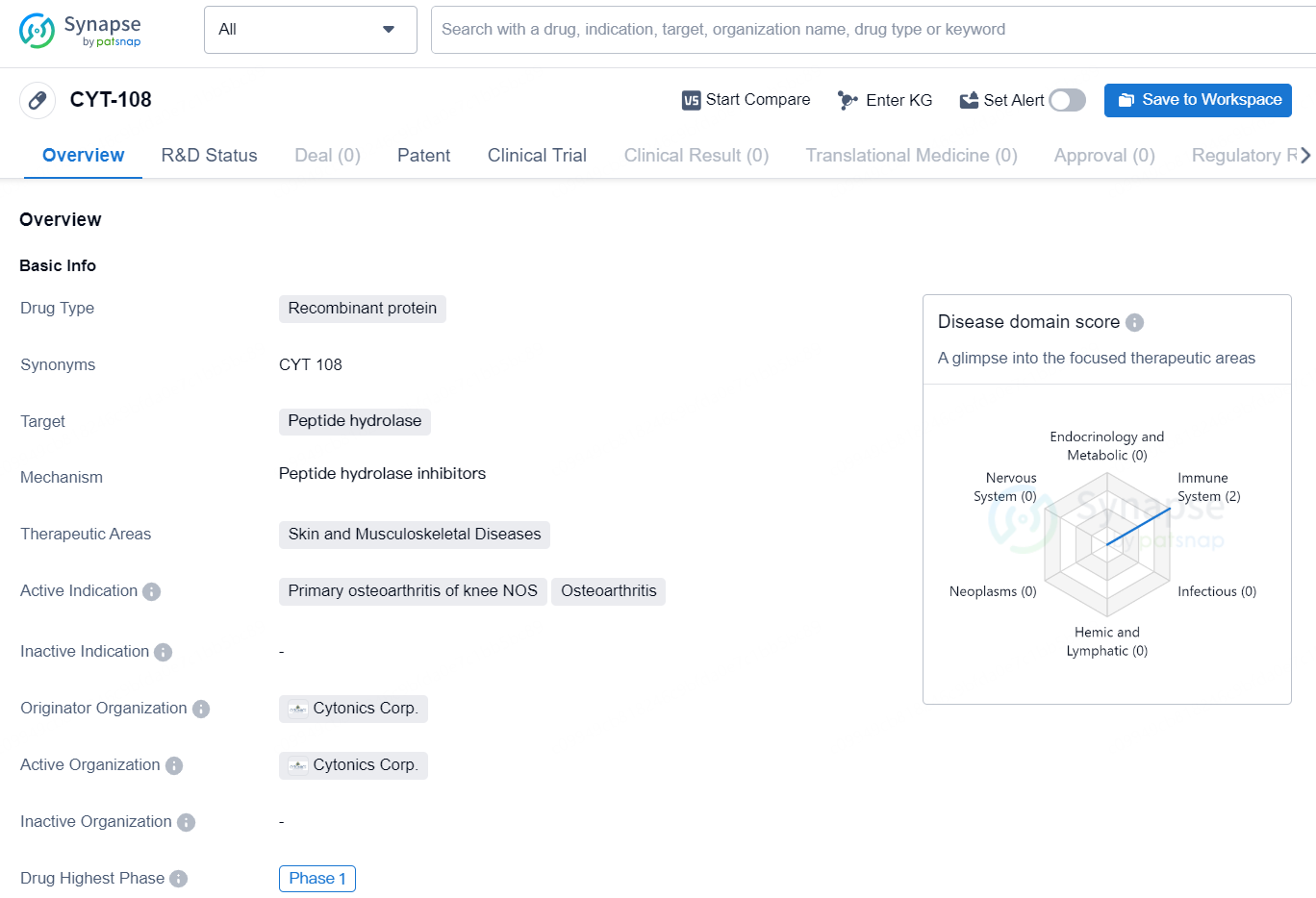
The drug CYT-108 is a recombinant protein that targets peptide hydrolase and is intended for use in treating skin and musculoskeletal diseases. Its active indications include primary osteoarthritis of the knee NOS and osteoarthritis. The drug is being developed by Cytonics Corp., and its highest phase of development globally is Phase 1. As a recombinant protein, CYT-108 is a therapeutic agent that has been engineered from genetic material to target specific biological processes. In this case, the drug targets peptide hydrolase, an enzyme involved in the breakdown of peptide bonds in proteins. By targeting this enzyme, it is expected that CYT-108 may have a beneficial effect on skin and musculoskeletal diseases, particularly in the treatment of primary osteoarthritis of the knee NOS and osteoarthritis.