Medigene's MDG1015, a Third-Generation T Cell Therapy, Wins FDA Approval for IND Trials in Solid Tumors
Medigene AG (Medigene or the "Company", FSE: MDG1, Prime Standard), a company specializing in oncology with a focus on the development and research of T cell receptor (TCR)-based therapies for cancer treatment, announced that the U.S. Food and Drug Administration (FDA) has approved the Company's Investigational New Drug (IND) application for its leading program MDG1015. This program aims to treat advanced gastric cancer, ovarian cancer, myxoid/round cell liposarcoma, and synovial sarcoma in the phase 1 clinical trial (EPITOME1015-I).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
EPITOME1015-I includes an initial dose-escalation phase succeeded by an expansion phase, with the primary objective to evaluate the safety, feasibility, and early efficacy of MDG1015 across various solid tumor types.
"We are thrilled to hit this significant milestone with our leading program MDG1015, which strengthens our goal of becoming a premier company offering a variety of TCR-guided therapies for patients with advanced solid tumors," stated Selwyn Ho, CEO of Medigene AG. "In preclinical trials, MDG1015 exhibited potent and sustained T cell-driven anti-tumor activity and the ability to counteract PD-L1-related immunosuppressive signals, which are prevalent in the tumor microenvironment of solid cancers and often impede the effectiveness of TCR-T therapies. This initial FDA clearance of an IND Application for a Medigene TCR-T therapy is a crucial advancement, and we are eager to launch our MDG1015 phase 1 study EPITOME1015-I aimed at multiple solid tumors, contingent upon further funding."
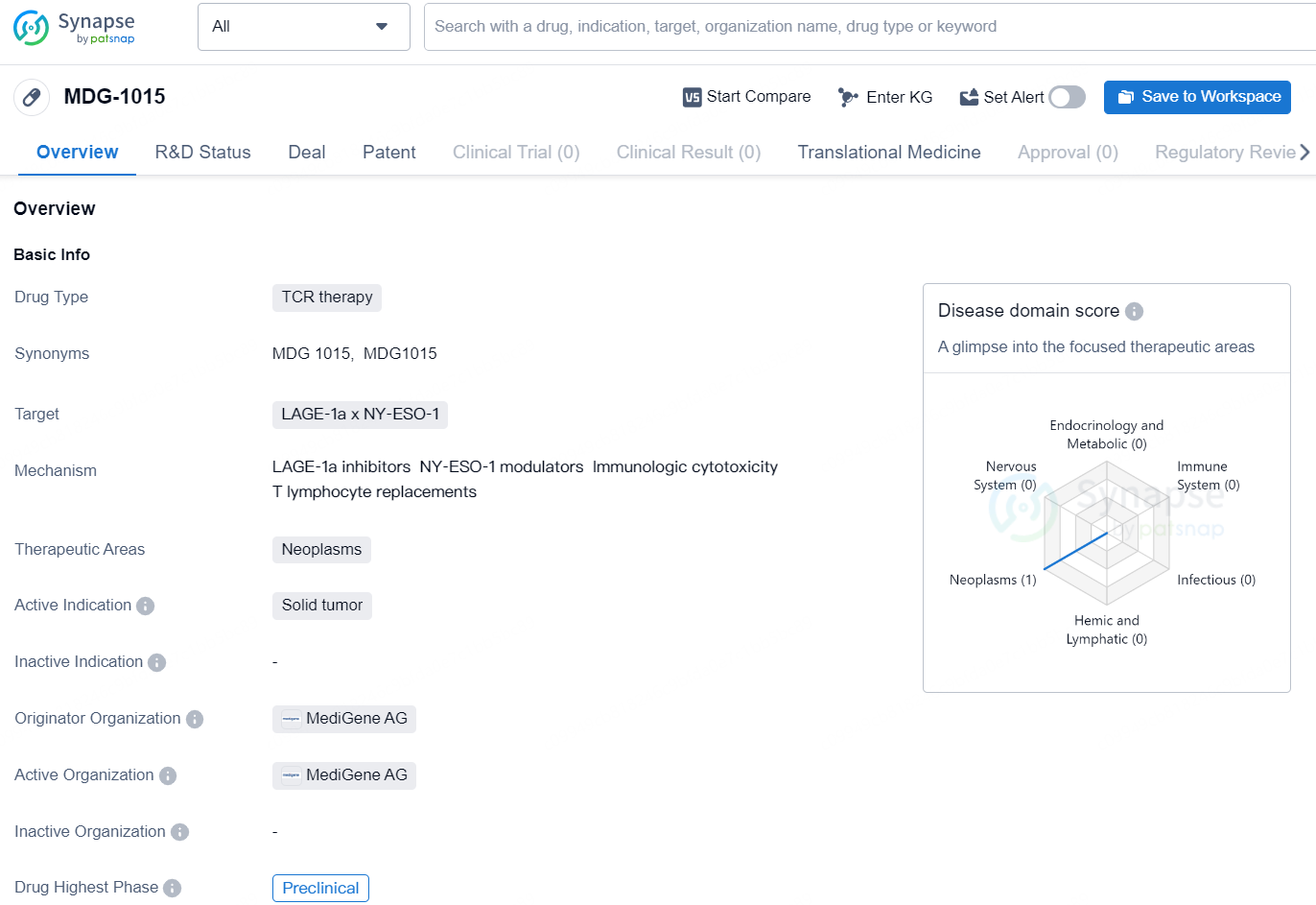
MDG1015 is a pioneering, third generation T cell receptor engineered T cell (TCR-T) therapy targeting the cancer-testis antigen New York esophageal squamous cell carcinoma 1 / L Antigen Family Member-1a (NY-ESO-1/LAGE-1a), featuring a natural and optimally affinity-balanced 3S (specific, sensitive, and safe) TCR and human leukocyte antigen (HLA)-A*02. These TCR-T cells are further enhanced by the proprietary PD1-41BB costimulatory switch protein (CSP) technology, enabling increased anti-tumor activity against tumor cells with varying PD-L1 levels, one of the most potent immunosuppressive signals in the solid tumor microenvironment.
Notably, compared to earlier generation TCR-T therapies, MDG1015 is manufactured with a brief, 6-day cell expansion period, resulting in younger, more robust cells, potentially lowering the required cell dose and shortening the vein-to-vein time for patients to approximately 20 days. This also produces a treatment with an almost pure CD8+ population and a high proportion of cells with stem-like properties (~95%), which may enhance response durability, efficacy, and reduce side effects.
In addition to the IND approval, a Clinical Trial Application (CTA) submission for MDG1015 to the European Medicines Agency (EMA) is projected for the fourth quarter of 2024. With adequate financing, the Company aims to commence the phase 1 clinical trial EPITOME-1015-I, consisting of dose escalation followed by an expansion segment, by late 2024. Following this schedule, the Company anticipates presenting initial data from the dose escalation phase by late 2025.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
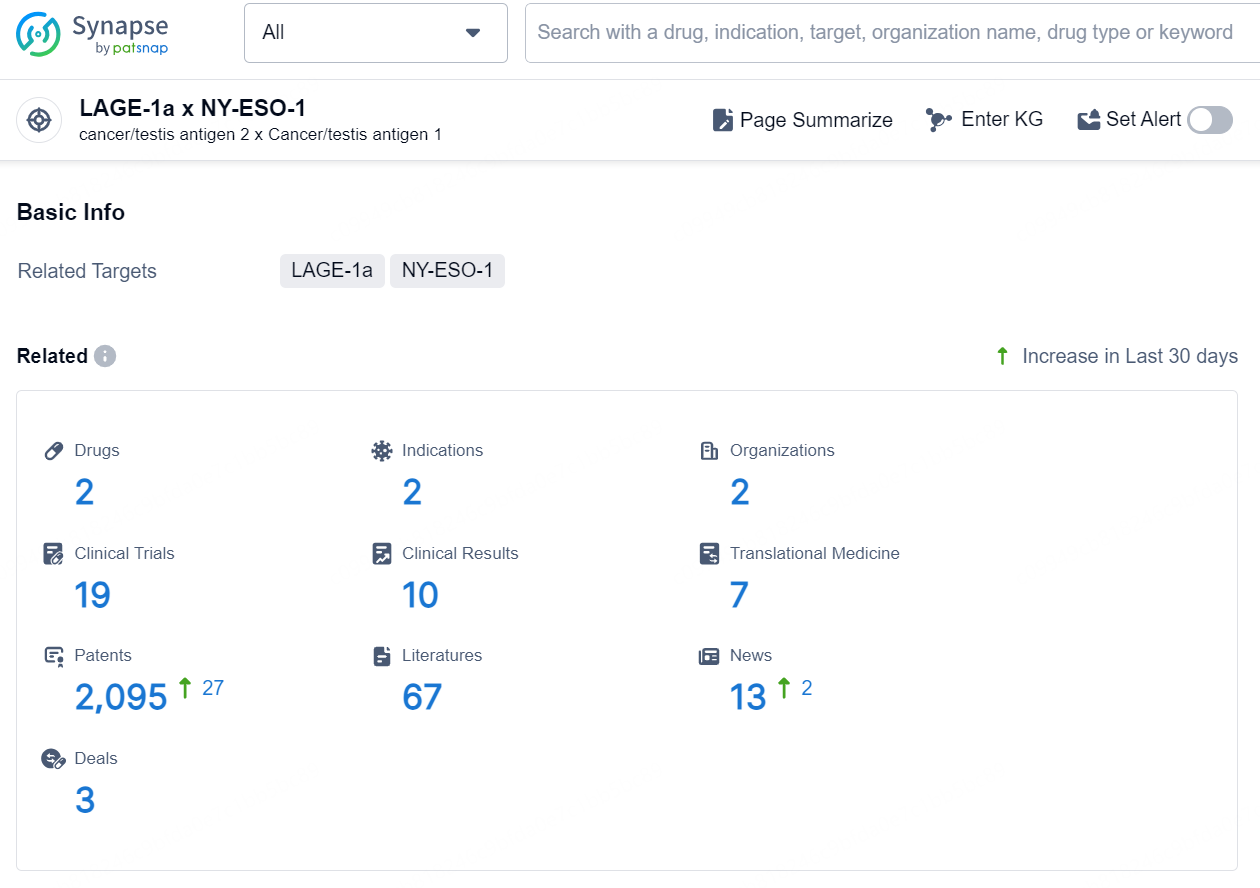
According to the data provided by the Synapse Database, As of September 9, 2024, there are 2 investigational drugs for the LAGE-1a x NY-ESO-1 target, including 2 indications, 2 R&D institutions involved, with related clinical trials reaching 19, and as many as 2095 patents.
MDG-1015 is a TCR therapy drug developed by MediGene AG. This drug targets LAGE-1a x NY-ESO-1 and is intended for the treatment of neoplasms, specifically solid tumors. At present, MDG-1015 is in the preclinical phase, which indicates that it is still undergoing early-stage testing and has not yet progressed to clinical trials. As a TCR (T cell receptor) therapy, MDG-1015 is designed to harness the power of the immune system to target and destroy cancer cells that express specific tumor-associated antigens, in this case, LAGE-1a and NY-ESO-1. TCR therapy works by genetically modifying a patient's T cells to express T cell receptors that are specific to these antigens, thus enabling the T cells to recognize and attack the cancer cells.