Datopotamab Deruxtecan: A Quick Look at Its R&D Progress and Clinical Results from the 2024 ASCO_GU
Datopotamab deruxtecan (Dato-DXd) is an antibody-drug conjugate consisting of a humanized anti-TROP2 IgG1 monoclonal antibody covalently linked to a highly potent topoisomerase I inhibitor payload via a plasma-stable, tumor-selective, tetrapeptide-based cleavable linker. Dato-DXd has shown encouraging antitumor activity and a manageable safety profile in patients (pts) with solid tumors. Recently, the clinical results in pts with advanced/metastatic (a/m) urothelial cancer from the ongoing phase 1 TROPION-PanTumor01 study (NCT03401385) were reported in 2024 ASCO_GU.
Datopotamab Deruxtecan's R&D Progress
Disitamab Vedotin is an antibody drug conjugate (ADC) and monoclonal antibody that targets HER2 and Tubulin. It has been approved for various therapeutic areas including neoplasms, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, other diseases, nervous system diseases, congenital disorders, eye diseases.
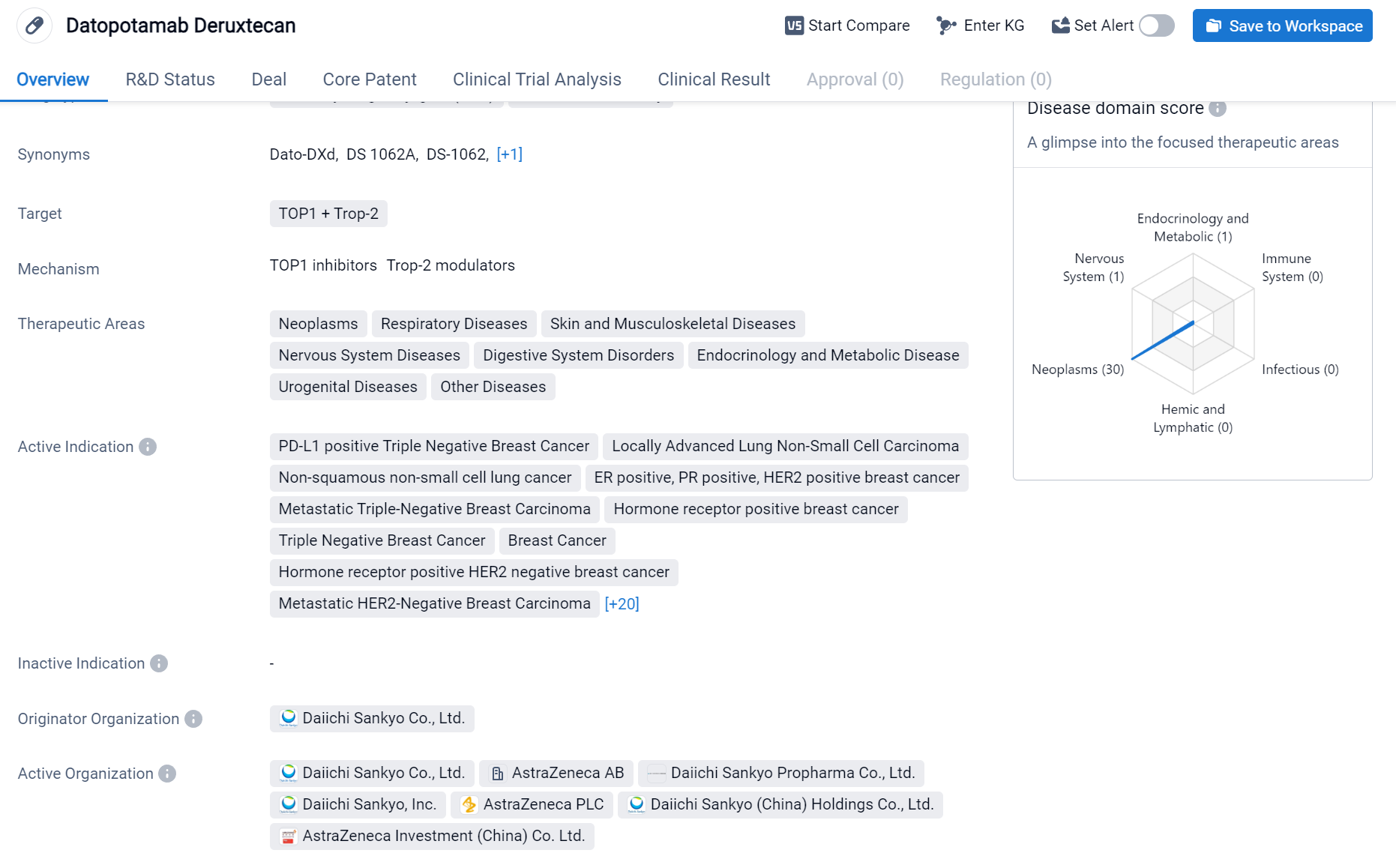
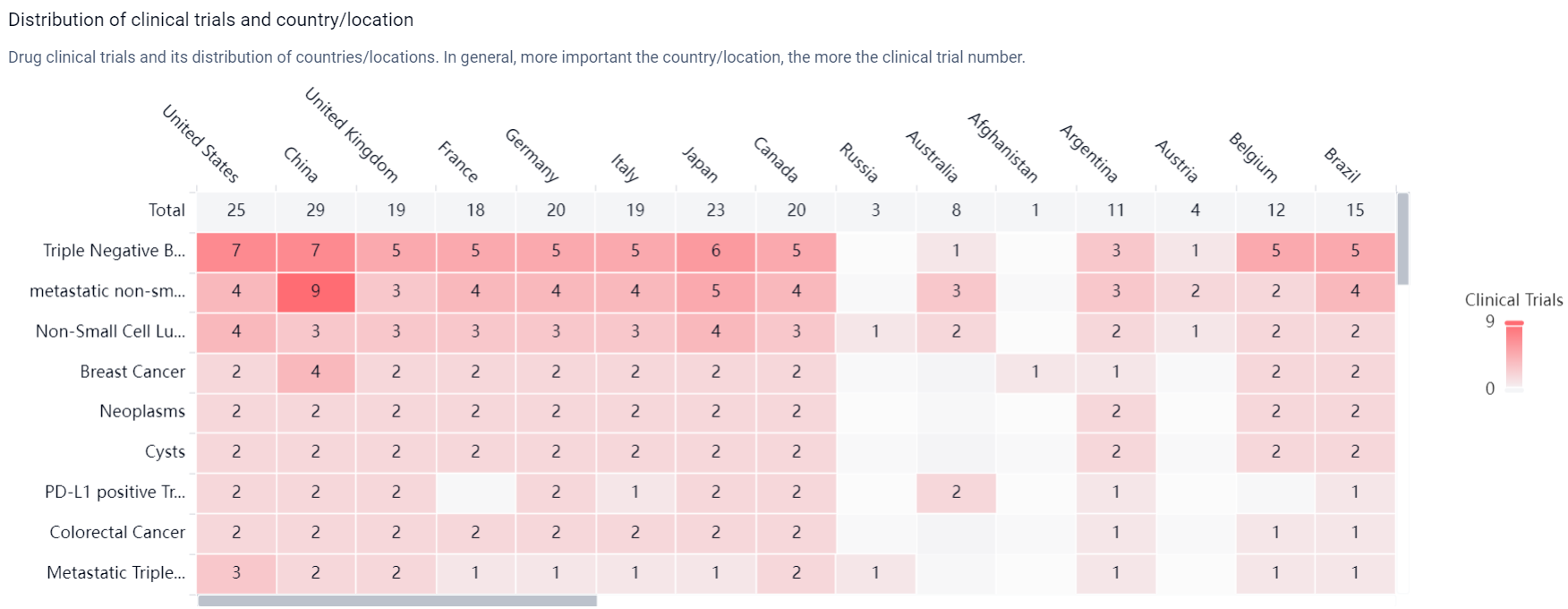
According to the Patsnap Synapse, Datopotamab Deruxtecan's highest phase of development is approved globally. And the clinical trial distributions for Datopotamab Deruxtecan are primarily in the United States, China and United Kingdom. The key indication is Triple Negative Breast Cancer.
Detailed Clinical Result of Datopotamab Deruxtecan
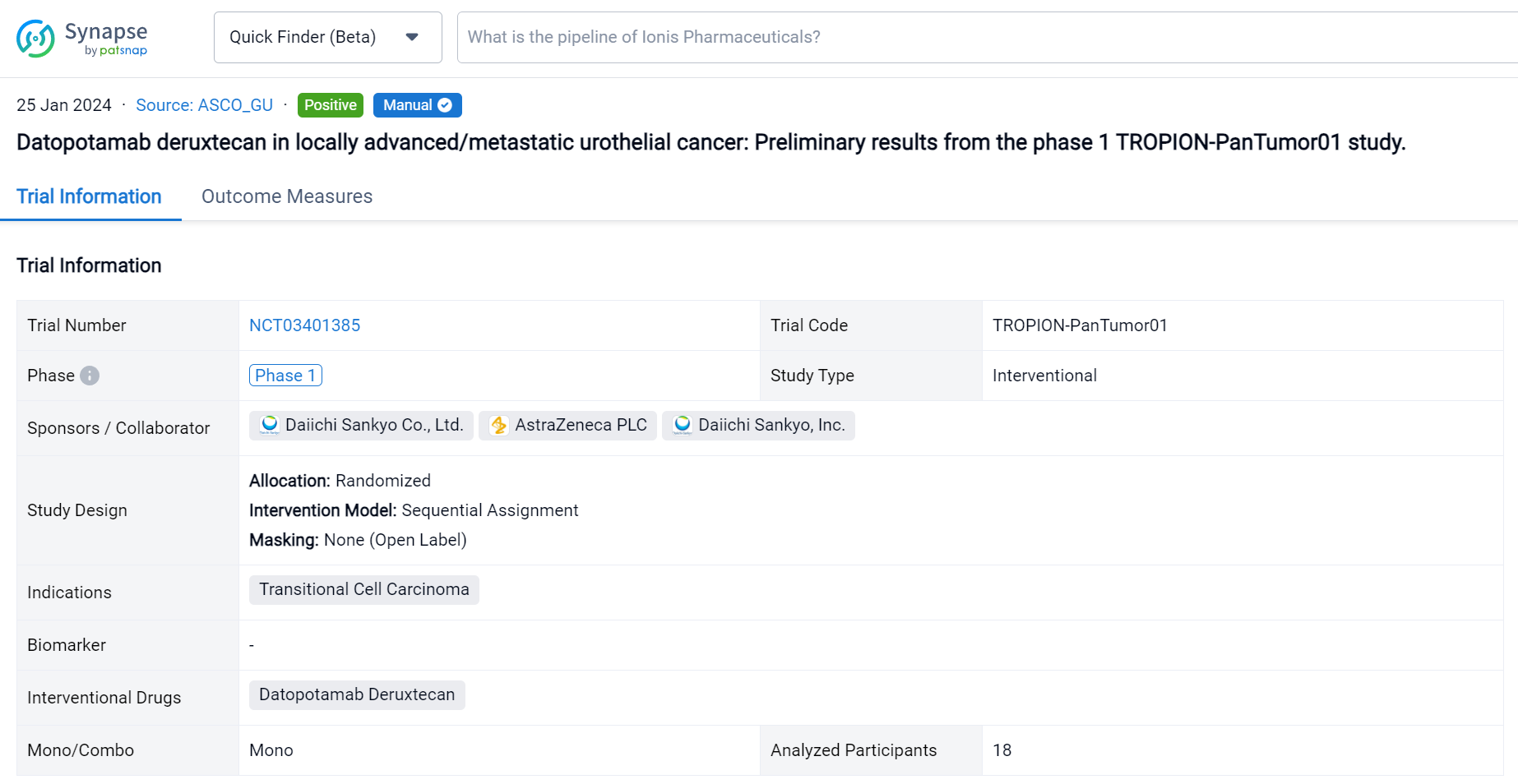
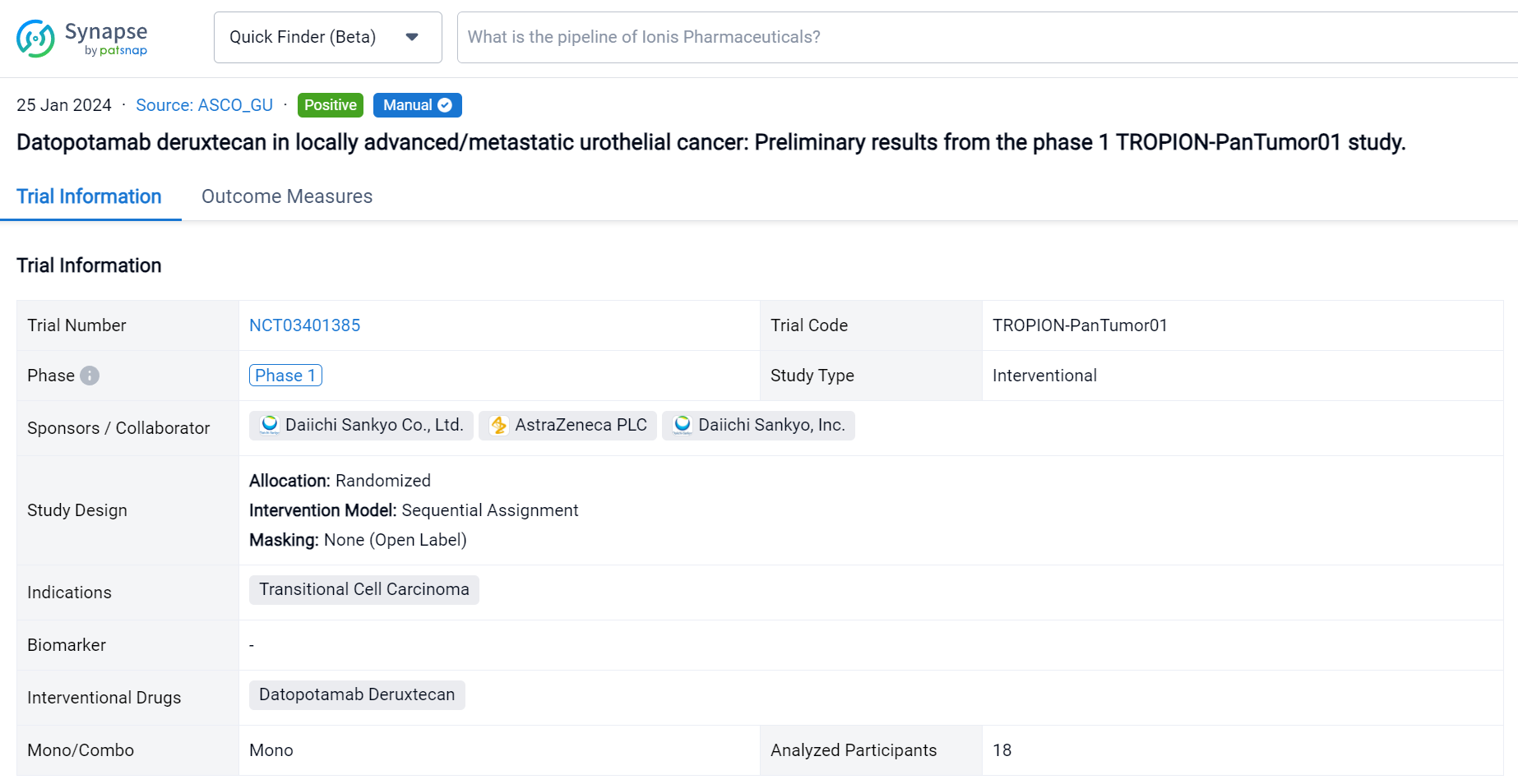
This randomized, sequential assignment, open-Labelled clinical trial (NCT03401385) was aimed to evaluate the safety and efficacy of Datopotamab Deruxtecan for the treatment of locally advanced/metastatic urothelial cancer.
In this study, pts with unresectable a/m urothelial cancer treated with ≥1 prior line of therapy received intravenous Dato-DXd 6 mg/kg Q3W. Primary study objectives were safety and tolerability. Secondary endpoints were objective response rate (ORR; complete response [CR] + partial response [PR]), and disease control rate (DCR; CR + PR + stable disease) per RECIST 1.1 by BICR. This is the first data disclosure from this cohort in an ongoing expansion cohort study.
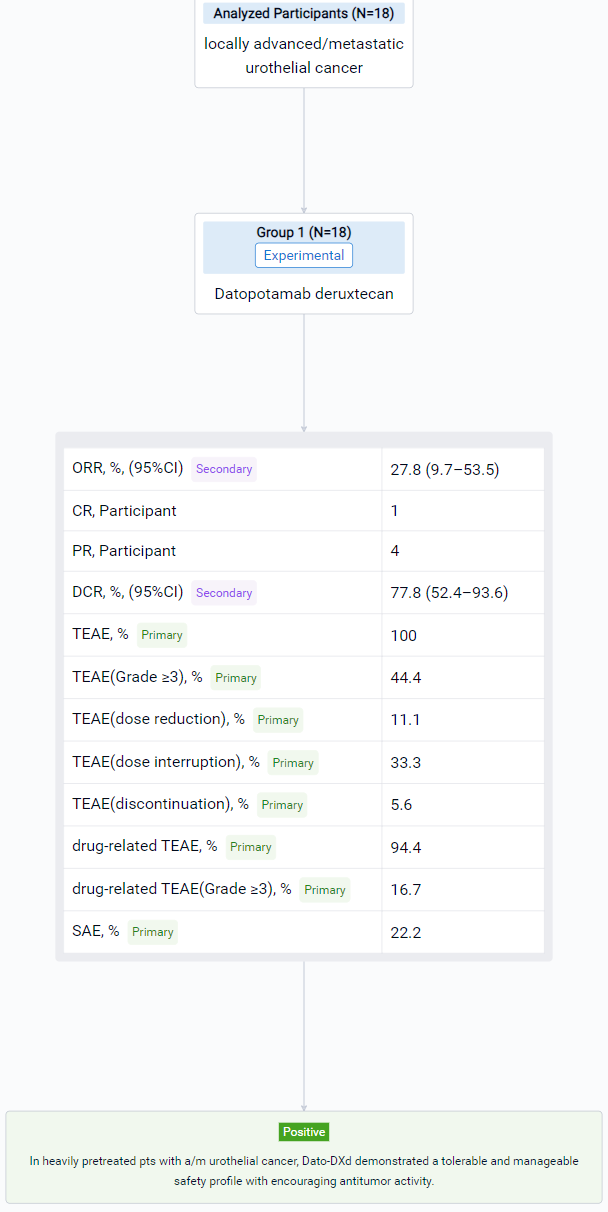
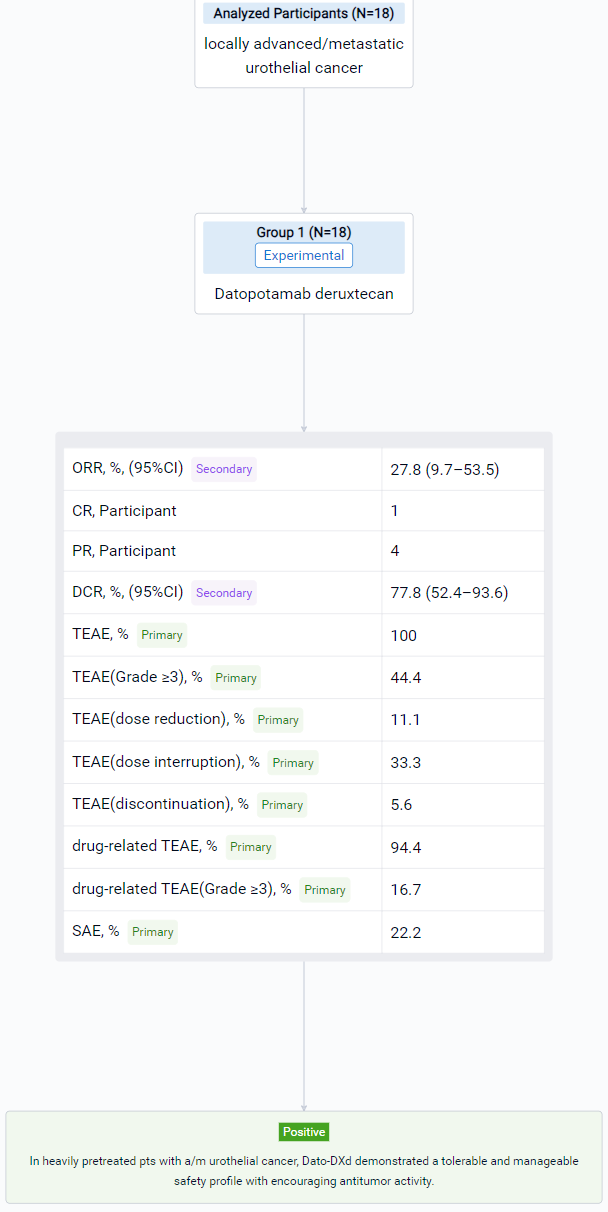
The result showed that At data cutoff (May 18, 2023), 18 pts had received Dato-DXd. Median follow-up was 9.1 (range 5–17) months; 6 (33.3%) pts were receiving ongoing treatment. Median age was 63.5 (range 46–79) yrs. Pts were heavily pretreated, 15 (83.3%) had received ≥3 prior regimens. All pts received prior immunotherapy, 17 (94.4%) prior platinum-based chemotherapy, and 4 (22.2%) prior taxanes. Treatment-emergent adverse events (TEAEs) occurred in 100% (any grade [gr]) and 44.4% (gr ≥3) of pts, and drug-related TEAEs occurred in 94.4% (any gr) and 16.7% (gr ≥3) of pts. No drug-related serious AEs were reported, and no TEAEs associated with death were observed. Any gr TEAEs associated with reduction, interruption, and discontinuation of treatment were reported in 11.1%, 33.3%, and 5.6% of pts, respectively (Table). Adjudicated drug-related interstitial lung disease (gr 2) occurred in 1 (5.6%) pt. Confirmed ORR was 27.8% (95% CI 9.7–53.5); 1 pt achieved a CR and 4 achieved a PR. DCR was 77.8% (95% CI 52.4–93.6). Clinical evaluation of this cohort is ongoing and updated results will be presented.
It can be concluded that in heavily pretreated pts with a/m urothelial cancer, Dato-DXd demonstrated a tolerable and manageable safety profile with encouraging antitumor activity. Dato-DXd is being evaluated in pts with urothelial cancer as part of the phase 1/2 TROPION-PanTumor02 (NCT05460273) and the phase 2 TROPION-PanTumor03 (NCT05489211) studies.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
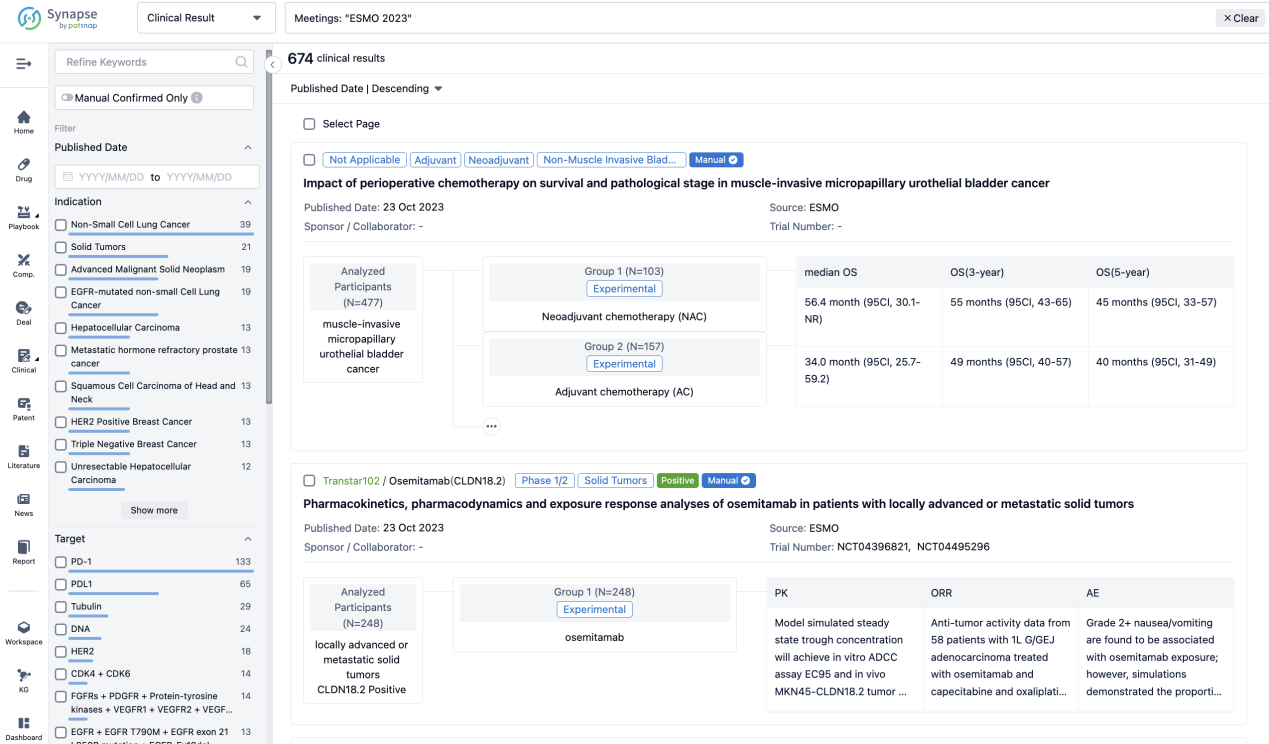
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
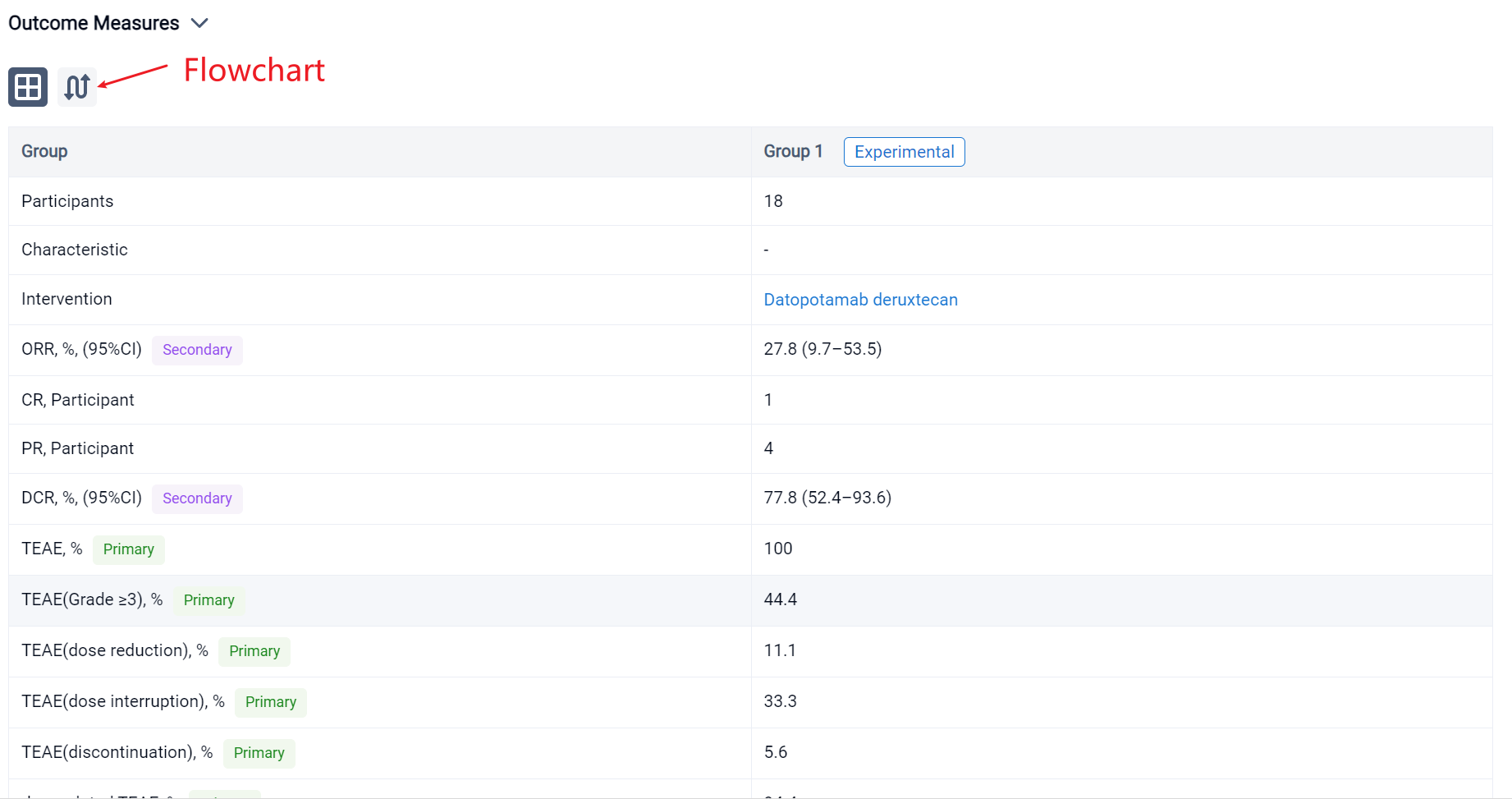
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
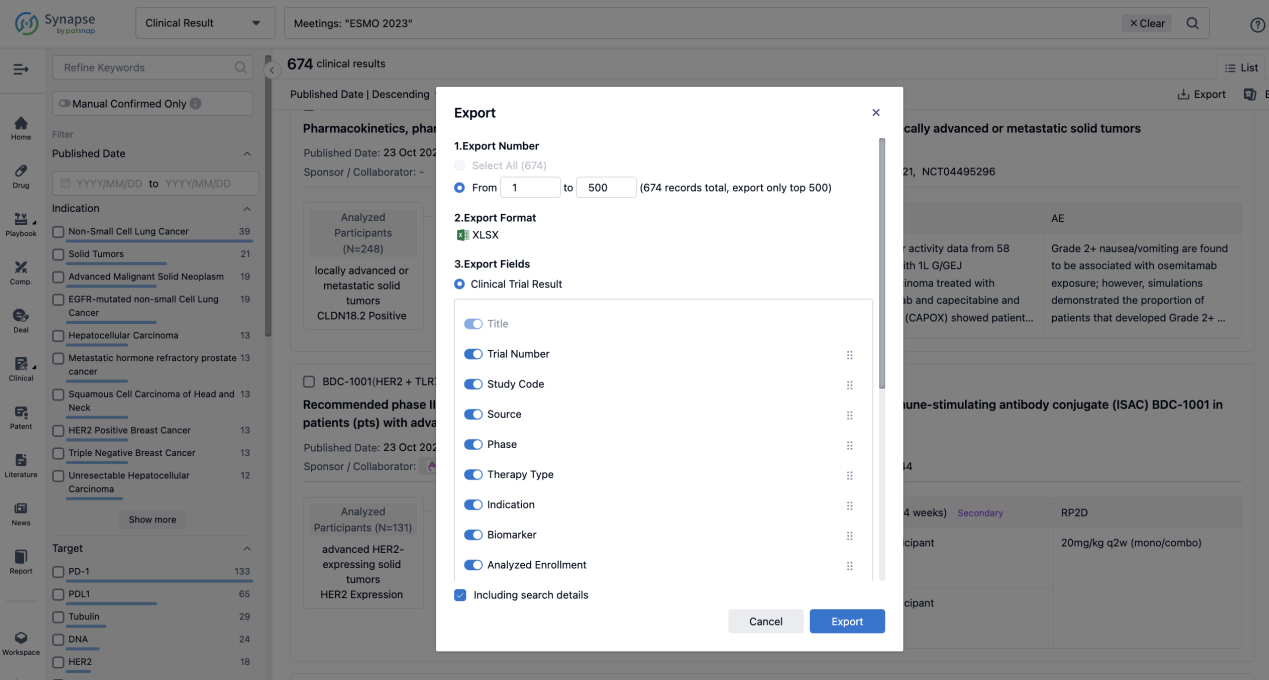
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!