Debiopharm and Repare Therapeutics partner to explore combined PKMYT1 and WEE1 inhibitor use for cancer therapy
Debiopharm, an independent biopharmaceutical firm headquartered in Switzerland, focuses on pioneering future norms for treating oncological and infectious conditions. The company has revealed a partnership and clinical trial agreement with Repare Therapeutics Inc., which is at the forefront of developing novel precision oncology treatments that are in the clinical trial phase.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
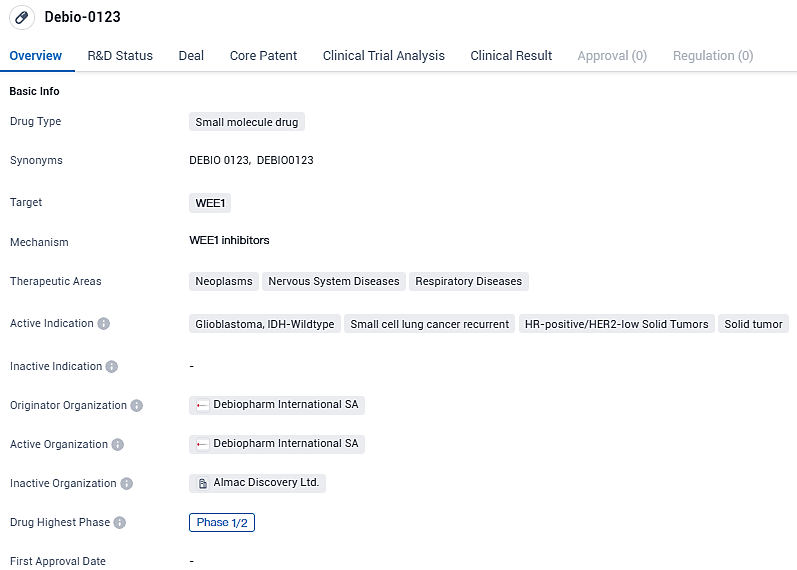
This joint medical venture is focused on assessing the combined effect of Debio 0123, a top-tier candidate known for its specificity, ability to cross the blood-brain barrier, and its role as a WEE1 inhibitor, alongside lunresertib, an unparalleled oral inhibitor of PKMYT1 which is both selective and potent, already proving its efficacy as an anti-cancer agent.
Per the agreement for the clinical collaboration and investigation, a new segment will be incorporated within the existing global MYTHIC study sponsored by Repare, designed to scrutinize the efficacy of the lunresertib and Debio 0123 union.
The commencement of the Phase 1/1b clinical investigation is projected for the latter part of 2024. Partners Debiopharm and Repare are set to co-create the specific trial arm framework to advance the combined treatment and will split all related expenses. Each entity will provide their unique therapeutic compounds to the study and maintain exclusive rights to commercialize their individual drugs, whether used in isolation or as part of combined treatment regimens.
"We're thrilled to launch this partnership with Repare, a pioneer in PKMYT1 inhibition, bolstering our dedication to advancing DDR therapies with our leading-edge WEE1 inhibitor," expressed Bertrand Ducrey, Debiopharm's CEO. "Through this approach rooted in synthetic lethality, we are poised to introduce a trailblazing targeted therapy for patients," added Ducrey, emphasizing the novelty of this venture in Debiopharm's strategy to pair two experimental drugs, showcasing the optimism surrounding this methodology for challenging cancer types.
Lloyd M. Segal, CEO of Repare, commented, "Integrating our efforts with Debiopharm’s exceptionally targeted WEE1 inhibitor aligns perfectly with our aspirations to push the boundaries of PKMYT1 inhibitor research." He also highlighted the solid scientific foundation and the encouraging preclinical evidence that both Repare and Debiopharm have accumulated, fostering trust in the approach to significantly improve treatment outcomes for patients facing limited therapeutic options.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
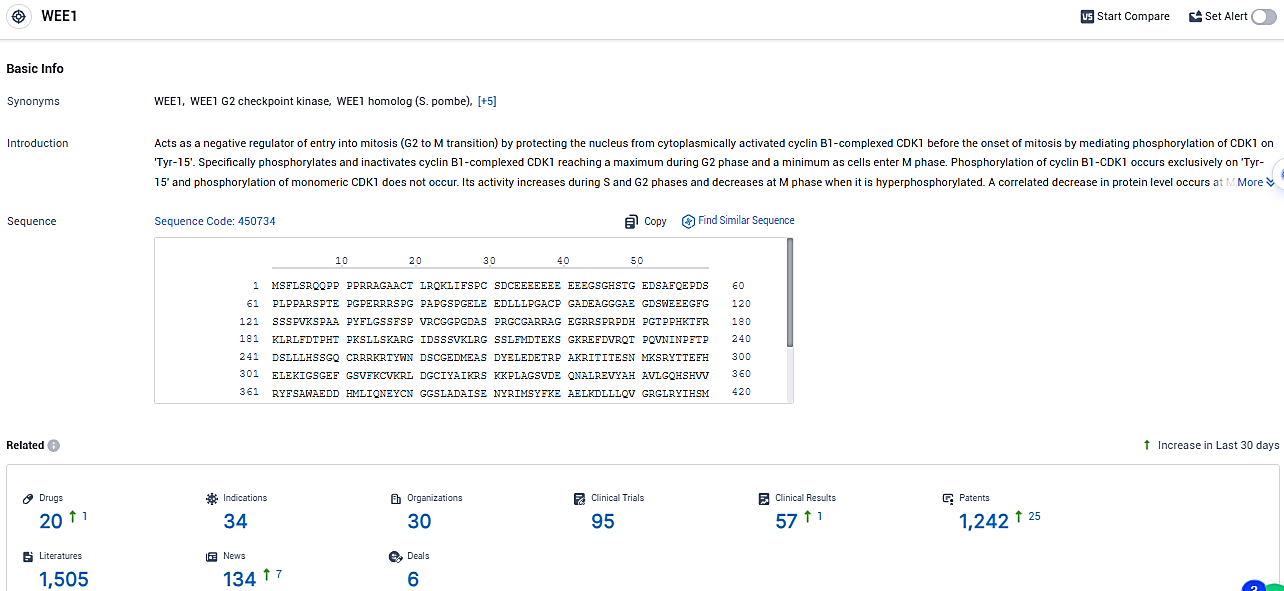
According to the data provided by the Synapse Database, As of January 9, 2024, there are 20 investigational drugs for the WEE1 target, including 34 indications, 30 R&D institutions involved, with related clinical trials reaching 95, and as many as 1242 patents.
Debio 0123 is a brain-penetrant, highly selective WEE1 kinase inhibitor. WEE1 is a key regulator of the G2/M and S phase checkpoints, activated in response to DNA damage, allowing cells to repair their DNA before resuming their cell cycle. Debio-0123 shows promise as a potential treatment for various types of cancers and other diseases within the neoplasms, nervous system diseases, and respiratory diseases therapeutic areas.