Kyverna's KYV-101 has received FDA clearance for a Phase 2 trial, KYSA-7, for advanced Multiple Sclerosis patients resistant to other treatments
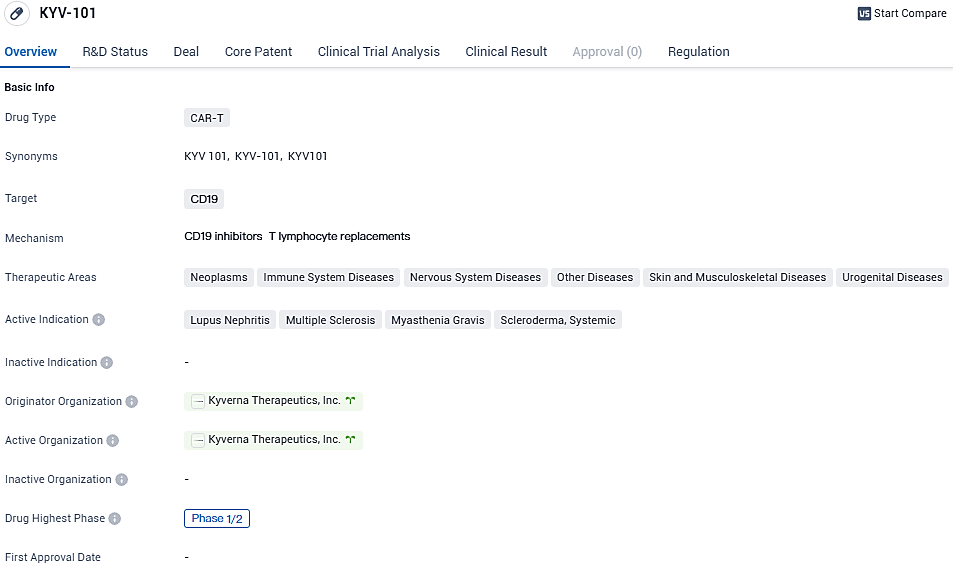
Kyverna Therapeutics, Inc., a biotechnology firm committed to advancing the field of cell-based treatments for individuals with autoimmune conditions, has publicized the approval of their IND submission by the FDA. This nod allows the progression of their proprietary, autologous CD19 CAR T-cell therapy, known as KYV-101, into clinical trials, with the aim of providing a new therapeutic option for the management of multiple sclerosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The greenlighting of this clinical phase is a vital milestone that sets the stage for enlisting treatment-resistant progressive MS patients in the KYSA-7 trial, with current therapies not addressing their condition. "This trial kindles new prospects for these individuals to halt further deterioration of their capabilities and possibly achieve sustained periods without the necessity of ongoing treatments," expressed Bruce Cree, M.D., Ph.D., MAS, the leading figure in clinical research and a distinguished neuroscience educator at the University of California, San Francisco, CA.
Manuel Friese, M.D., a respected neurological professor and head of the Institute of Neuroimmunology and Multiple Sclerosis at University Medical Center Hamburg-Eppendorf in Hamburg, Germany, noted, "It's crucial for this trial to ascertain the viability of CAR T-cell therapy as a novel remedy for those diagnosed with MS. This treatment could significantly remodel the management of MS by essentially recalibrating the immune response."
Leading the helm of a patient-focused entity, Peter Maag, Ph.D., CEO of Kyverna, expressed exhilaration for the wider patient inclusion facilitated by KYV-101 in the KYSA-7 study, highlighting this as a notable shift in autoimmune disease management, reflecting the company's pledge to broaden the reach of transformative treatment options to a range of medical contexts.
CAR T-cell therapy is an advanced approach where a patient's T cells are re-engineered to target and eliminate B cells within their own system. Focusing on CD19, a protein present on B cells implicated in various autoimmune conditions, Kyverna’s CD19 CAR T-cell therapy, KYV-101, is at the forefront of this strategy. Kyverna anticipates further investigation into other potential uses for KYV-101 and to cultivate a strong portfolio of innovative immunotherapy candidates, striving to fulfill the unaddressed health demands in the realm of autoimmune disorders.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
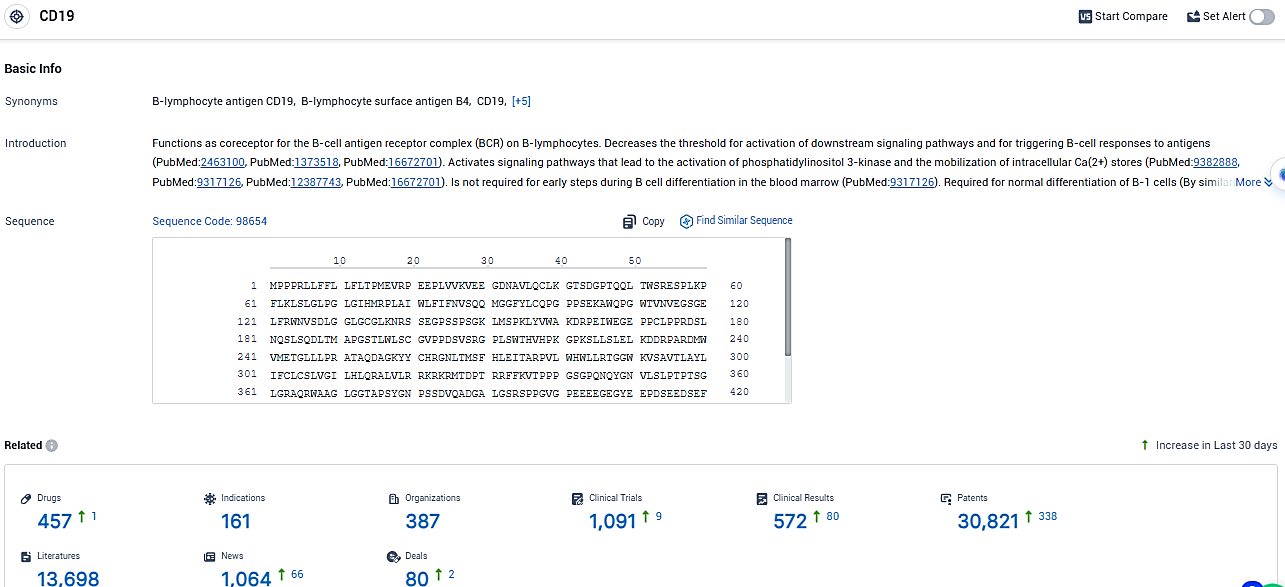
According to the data provided by the Synapse Database, As of January 9, 2024, there are 457 investigational drugs for the CD19 target, including 161 indications, 387 R&D institutions involved, with related clinical trials reaching 1091, and as many as 30821 patents.
KYV-101 is an autologous, fully human CD19 CAR T-cell product candidate for use in B cell-driven autoimmune diseases. Kyverna is currently conducting trials of KYV-101 in patients with lupus nephritis, an autoimmune disease in which more than half of patients do not achieve a complete response to current therapies and are at risk of developing kidney failure. Additional clinical trials of KYV-101 in systemic sclerosis, and myasthenia gravis are in preparation.