Decoding Axatilimab: a comprehensive study of its R&D trends and its clinical results in 2023 ASH
Chronic graft-versus-host disease (cGVHD) is an immune-mediated complication of allogeneic hematopoietic cell transplant (alloHCT) that affects multiple organs with inflammatory and fibrotic pathology. On 11 Dec 2023, the updated clinical results of Axatilimab at 3 different doses in patients with Chronic Graft-Versus-Host Disease (AGAVE-201) will be reported in 2023 ASH.
Axatilimab's R&D Progress
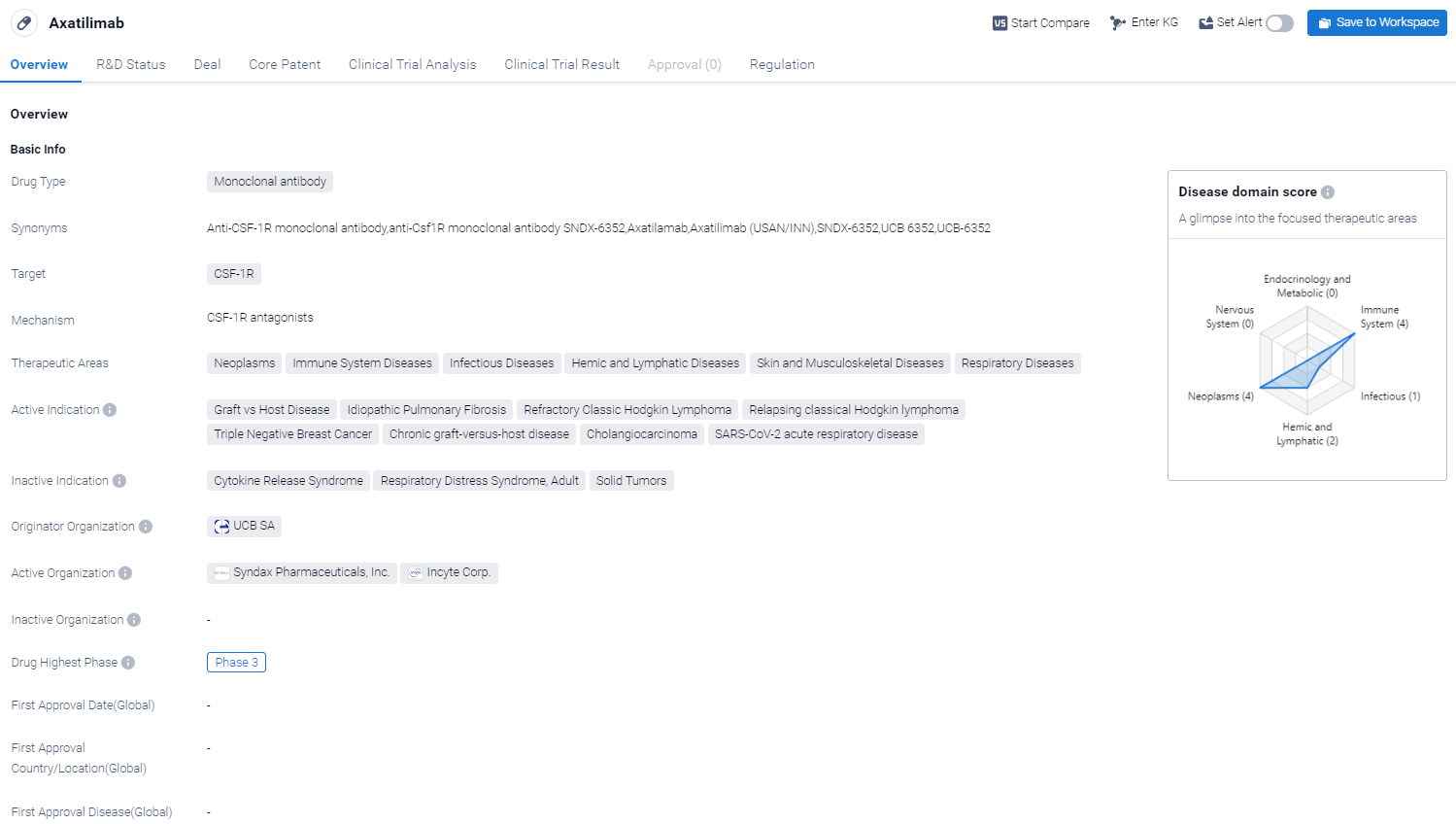
Axatilimab is a monoclonal antibody drug that targets CSF-1R, a receptor involved in various biological processes. It has shown potential therapeutic benefits in multiple therapeutic areas, including neoplasms, immune system diseases, infectious diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and respiratory diseases.
According to the Patsnap Synapse, Axatilimab has reached the highest phase of clinical development, which is Phase 3. And the clinical trial areas for Axatilimab are primarily in the United States, China and United Kingdom. The key indication is Unresectable Malignant Solid Neoplasm. 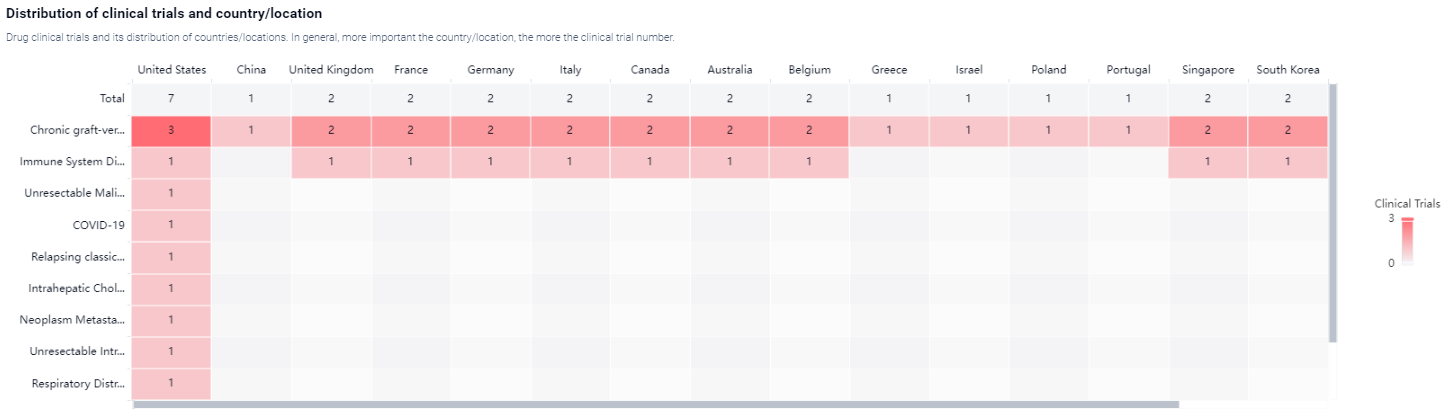
Detailed Clinical Result of Axatilimab
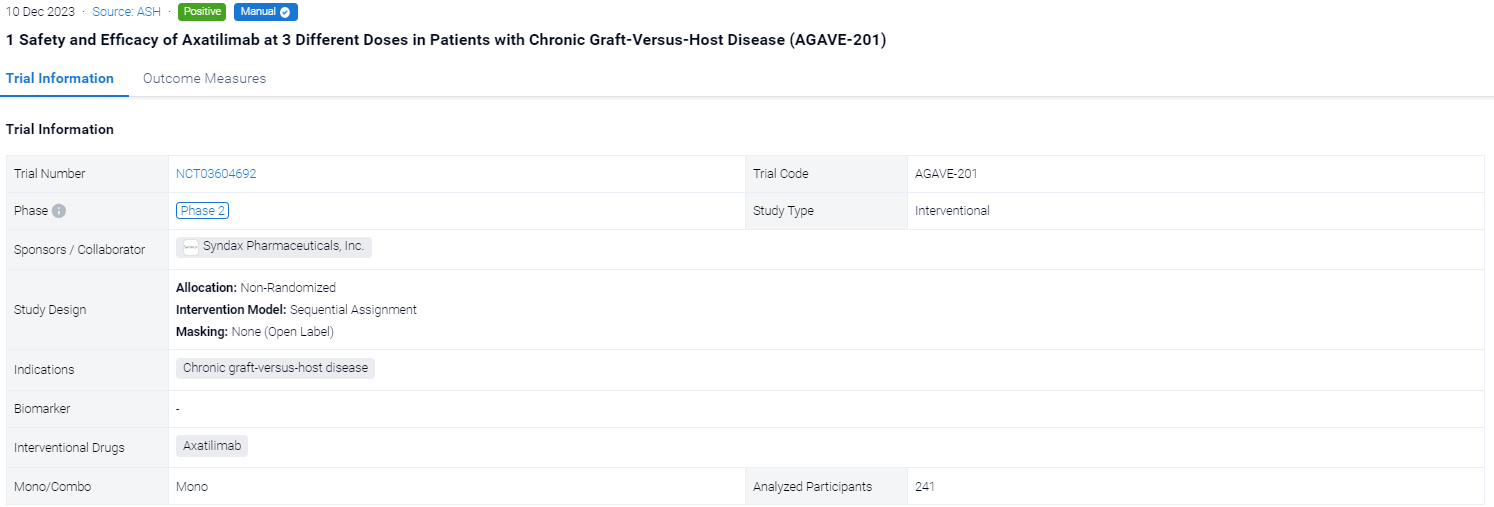
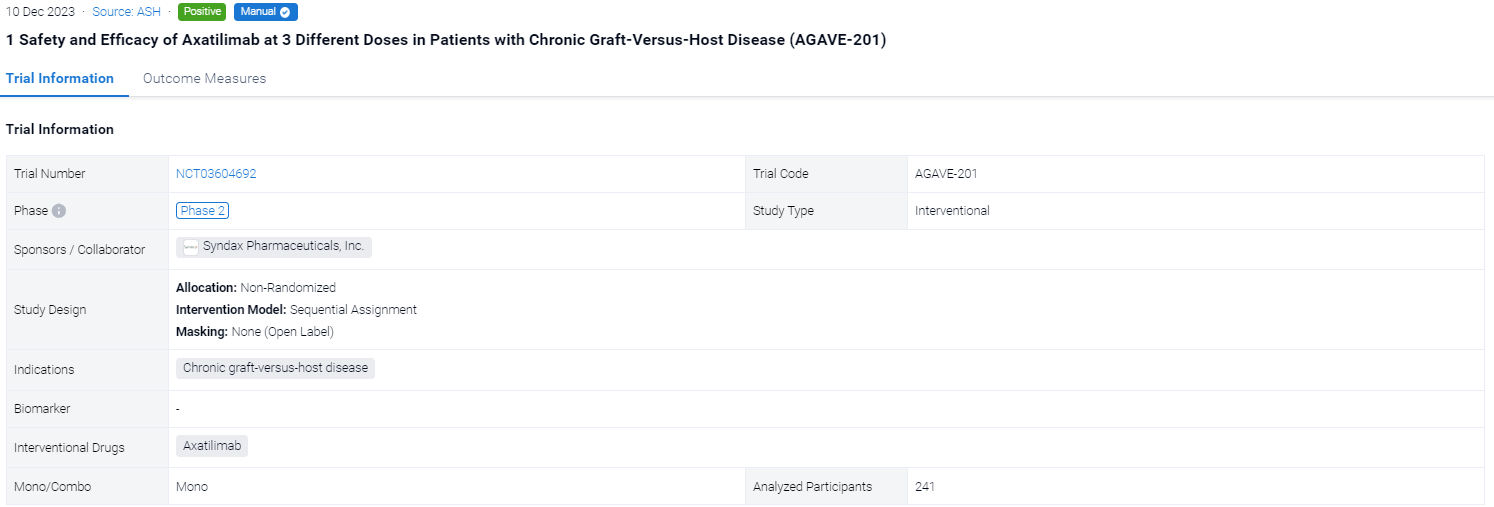
This pivotal phase 2, open-label, randomized, multicenter study (NCT03604692) investigate the safety and efficacy of Axatilimab at 3 different doses in alloHCT patients with recurrent or refractory cGVHD.
In this study, eligible patients were randomized 1:1:1 to receive intravenous (IV) axatilimab at 0.3 mg/kg every 2 weeks (Q2W), 1 mg/kg Q2W, or 3 mg/kg every 4 weeks (Q4W). Randomization was stratified by severity of cGVHD and prior use of ibrutinib, ruxolitinib, or belumosudil. Concomitant use of corticosteroids, calcineurin inhibitors, or mammalian target of rapamycin inhibitors (sirolimus or everolimus) was allowed. Axatilimab treatment could be continued as long as there was clinical benefit as assessed by the investigator. The primary efficacy endpoint was overall response rate (ORR) in the first 6 cycles (24 weeks) as defined by the NIH 2014 consensus criteria; the efficacy boundary of the ORR is based on the lower bound of the 95% CI exceeding 30%. The key secondary endpoint was the proportion of patients reporting a clinically significant reduction of symptoms, as measured by the modified Lee Symptom Scale (mLSS) score with a threshold of ≥7 points. Safety endpoints included frequency and severity of treatment-related adverse events (TRAEs) and treatment-emergent adverse events (TEAEs).
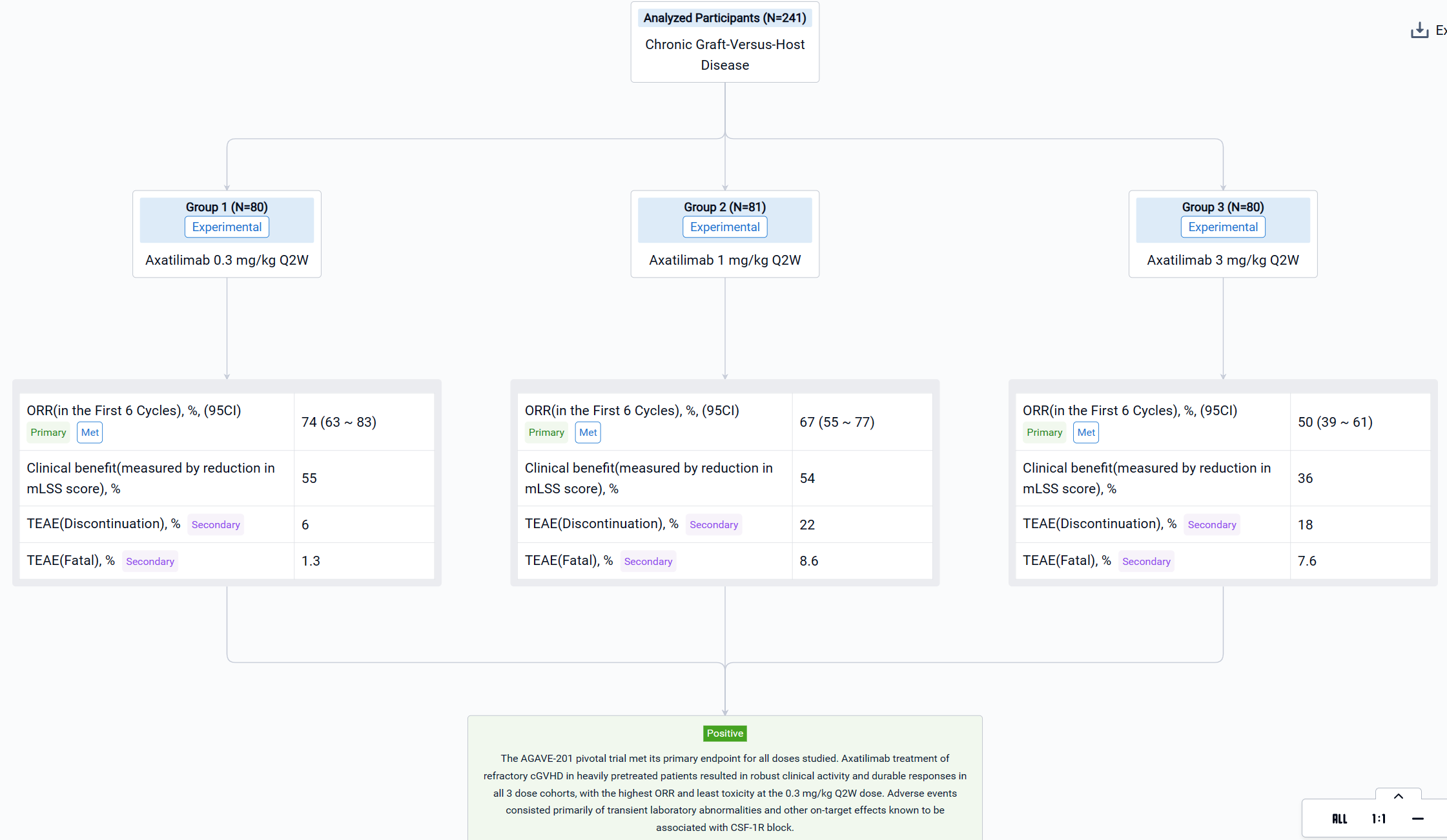
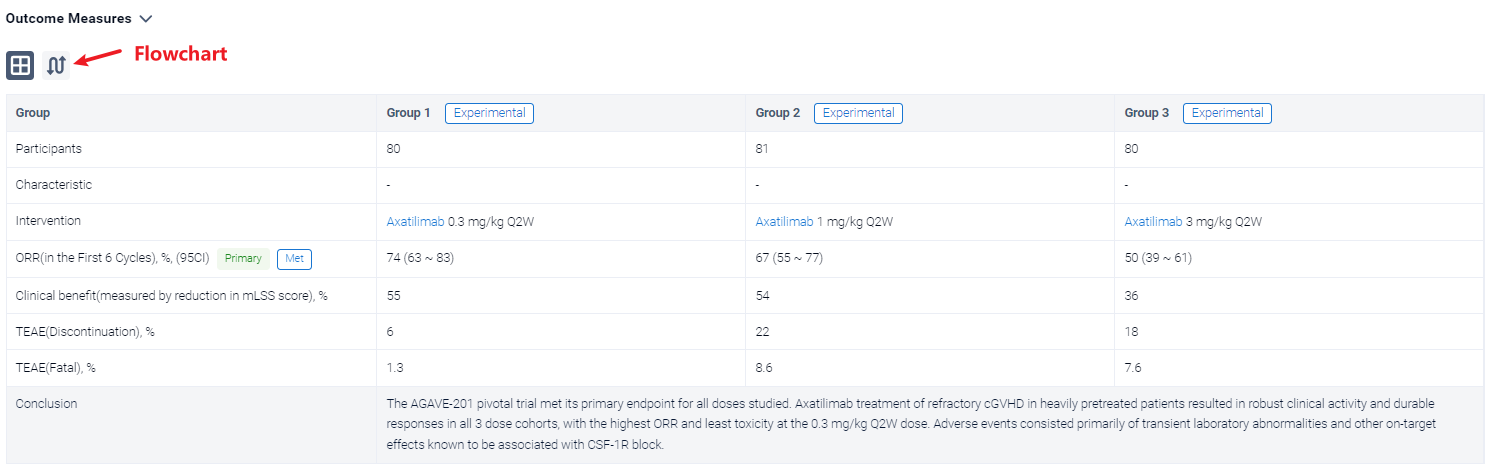
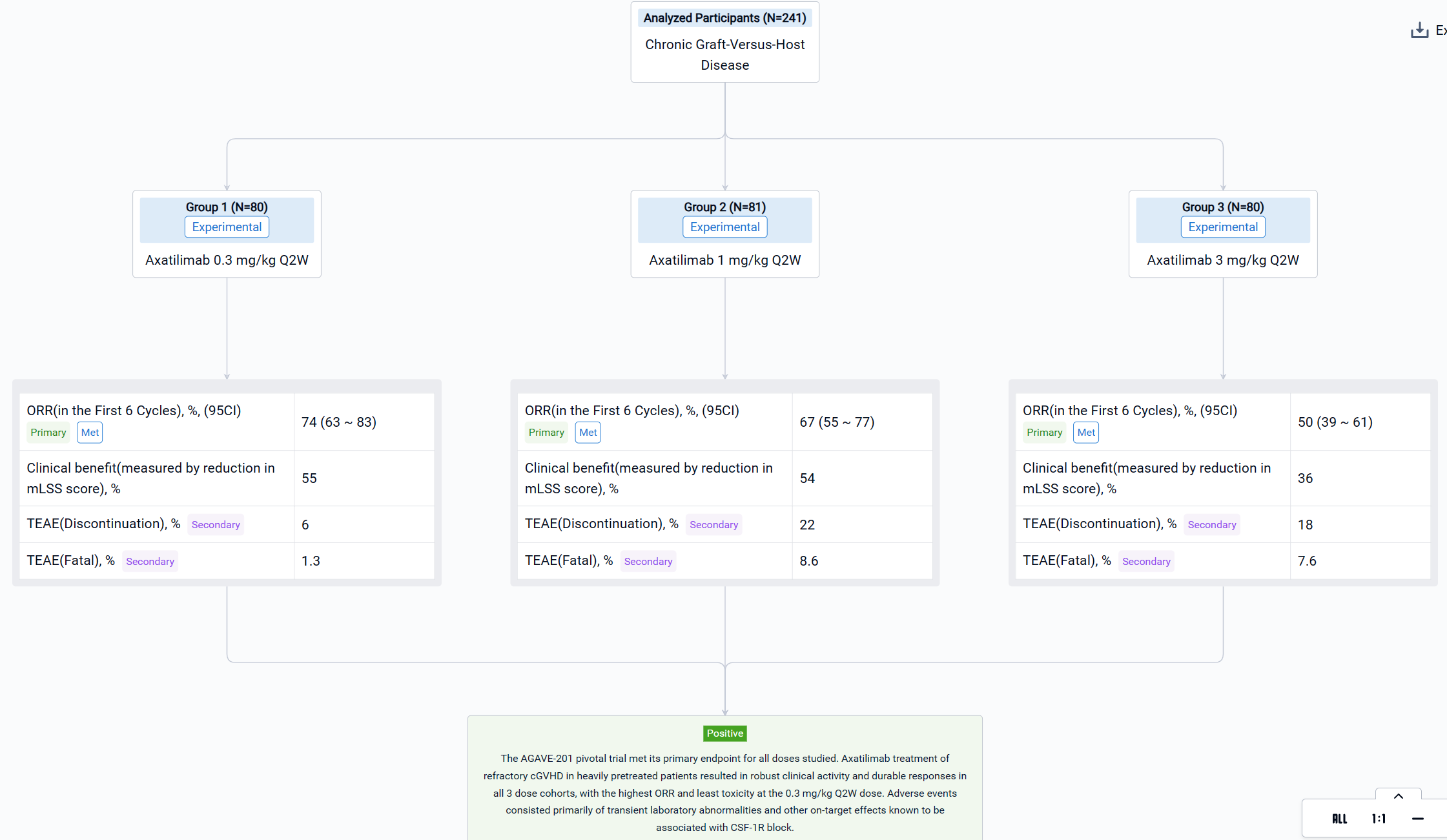
The result showed that a total of 241 patients were enrolled across 121 study sites and 239 (99.2%) patients were treated with axatilimab at the data cutoff of 7 April 2023. Patients were heavily pretreated with a median of 4 prior lines of therapy, including ruxolitinib (74%), belumosudil (23%), and ibrutinib (31%). Demographics and disease characteristics were balanced among the 3 dose cohorts (Table 1). ORR (95% CI) was 74% (63, 83) with 0.3 mg/kg Q2W, 67% (55, 77) with 1 mg/kg Q2W, and 50% (39, 61) with 3 mg/kg Q4W (Table 1). Median duration of response (DOR) has not been reached in any of the cohorts, with 60%, 60% and 53% of patients maintaining response at 12 months in the 0.3 mg/kg Q2W, 1 mg/kg Q2W, and 3 mg/kg Q4W dose cohorts, respectively. Clinical benefit, as measured by reduction in mLSS score, was reported in 55%, 54%, and 36% of patients in the 0.3 mg/kg Q2W, 1 mg/kg Q2W, and 3 mg/kg Q4W cohorts, respectively, in the first 6 cycles. Drug discontinuation owing to TEAEs occurred in 6% of patients with 0.3 mg/kg Q2W, 22% with 1 mg/kg Q2W, and 18% with 3 mg/kg Q4W. The most common TEAEs are summarized in Table 2. Fatal TEAEs occurred in 1.3%, 8.6%, and 7.6% of patients in the 0.3 mg/kg Q2W, 1 mg/kg Q2W, and 3 mg/kg Q4W dose cohorts, respectively. The frequency of TRAEs and grade ≥ 3 TRAEs were dose dependent, consistent with CSF-1R inhibition-mediated macrophage clearance. Most infections were mild, with 3 reported cytomegalovirus infections, including reactivations, in the higher dose cohorts.
It can be concluded that the AGAVE-201 pivotal trial met its primary endpoint for all doses studied. Axatilimab treatment of refractory cGVHD in heavily pretreated patients resulted in robust clinical activity and durable responses in all 3 dose cohorts, with the highest ORR and least toxicity at the 0.3 mg/kg Q2W dose. Adverse events consisted primarily of transient laboratory abnormalities and other on‑target effects known to be associated with CSF-1R block.
How to Easily View the Clinical Results Using Synapse Database?
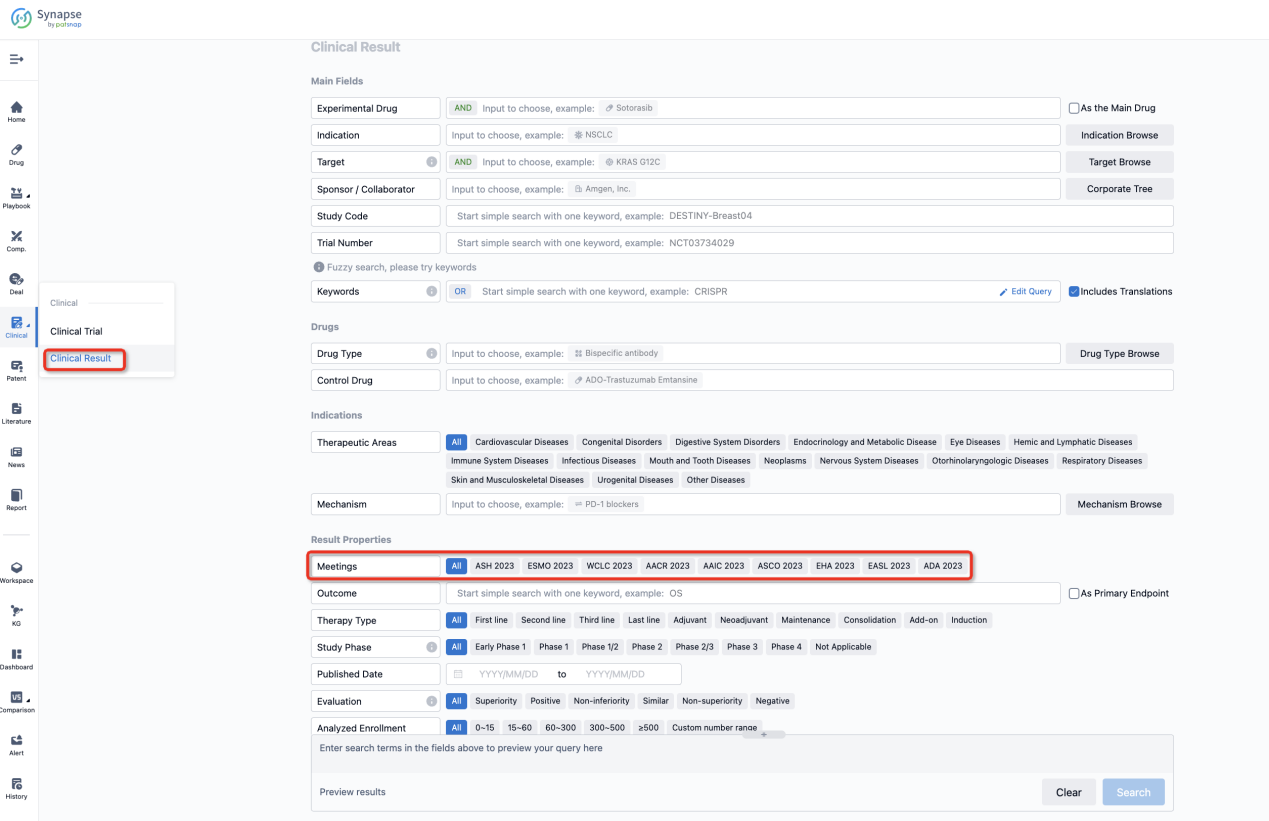
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
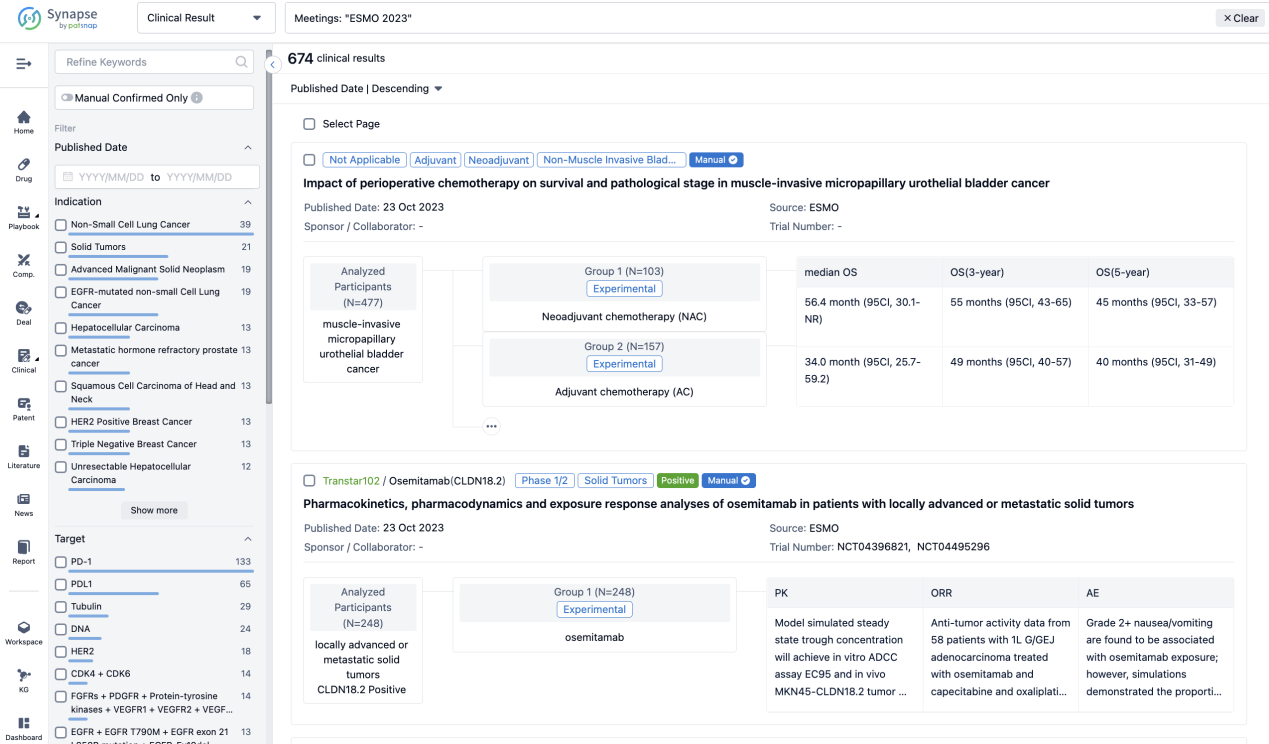
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
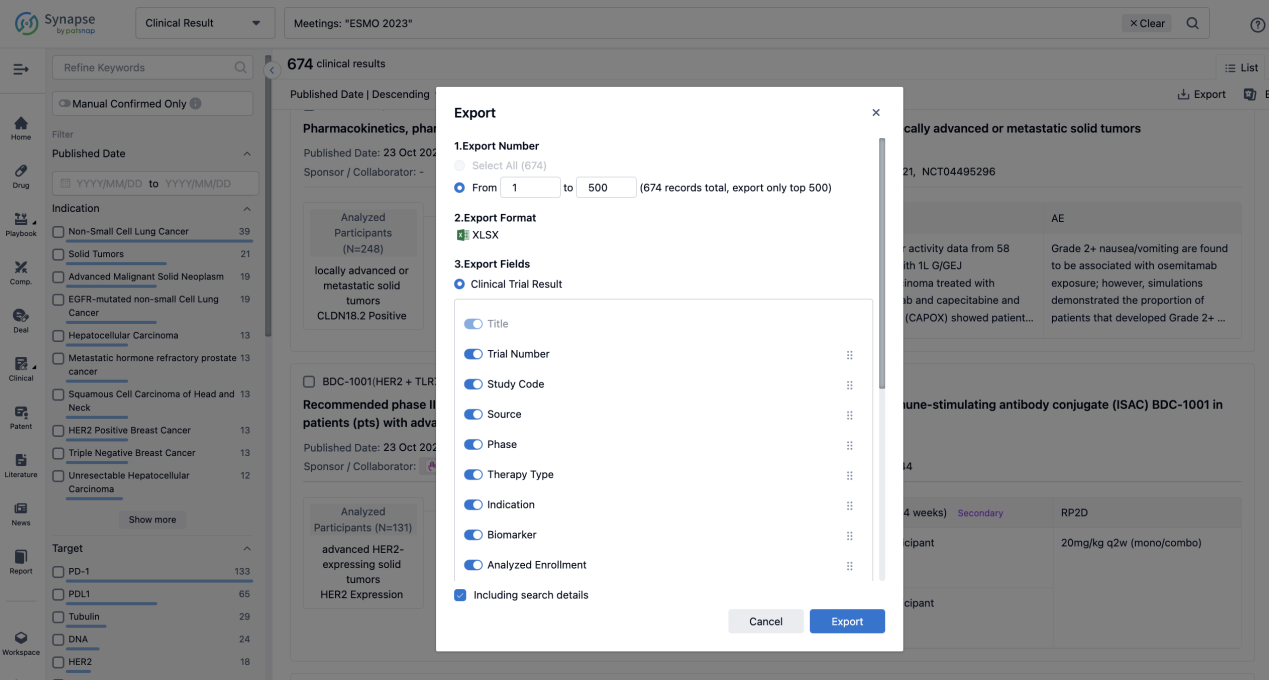
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!