Decoding Darolutamide: a comprehensive study of its R&D trends and its clinical results in 2024 ASCO_GU
On 25 Jan 2024, the rate of hospitalization and length of hospital stay during and post docetaxel for darolutamide in metastatic hormone-sensitive prostate cancer (darolutamide (daro) + docetaxel + androgen deprivation therapy (ADT) vs. placebo (pbo) + docetaxel + ADT) was reported in the 2024 ASCO_GU.
Darolutamide's R&D Progress
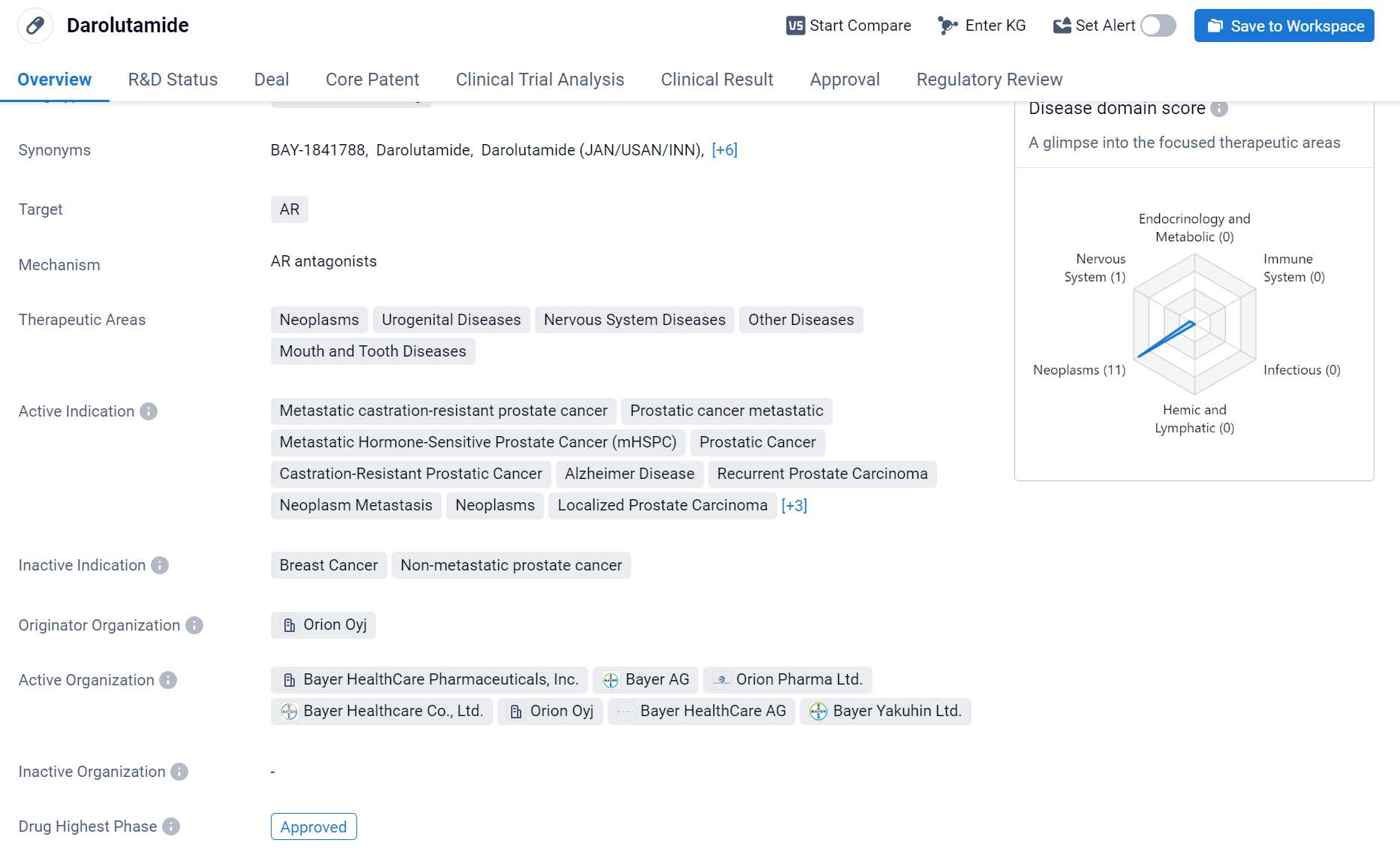
Darolutamide is a small molecule drug that targets the androgen receptor (AR). It has been approved for use in various therapeutic areas, including neoplasms, urogenital diseases, nervous system diseases, other diseases, mouth and tooth diseases.
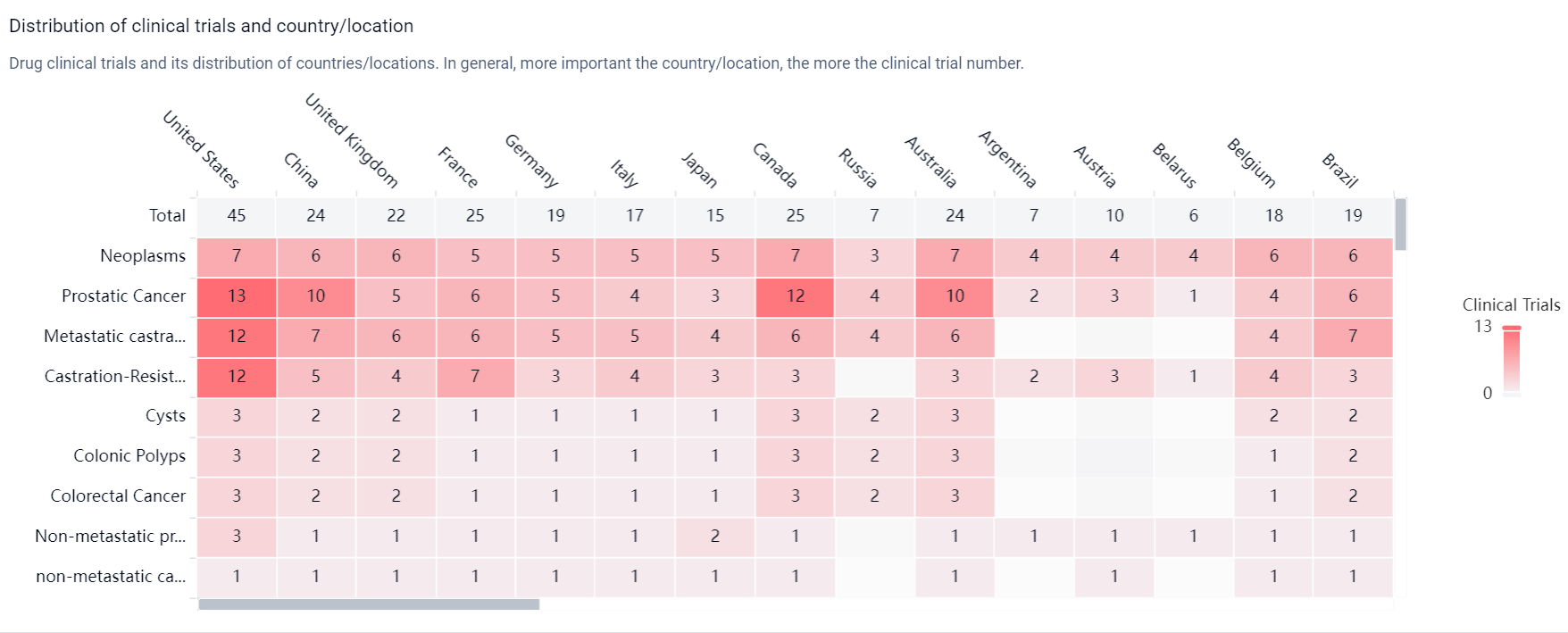
According to the Patsnap Synapse, Darolutamide was developed by Orion Oyj and has received approval in multiple countries. The highest phase of development for this drug is approved. And the clinical trial distributions for Darolutamide are primarily in the United States, China and United Kingdom. The key indication is Neoplasms.
Detailed Clinical Result of Darolutamide
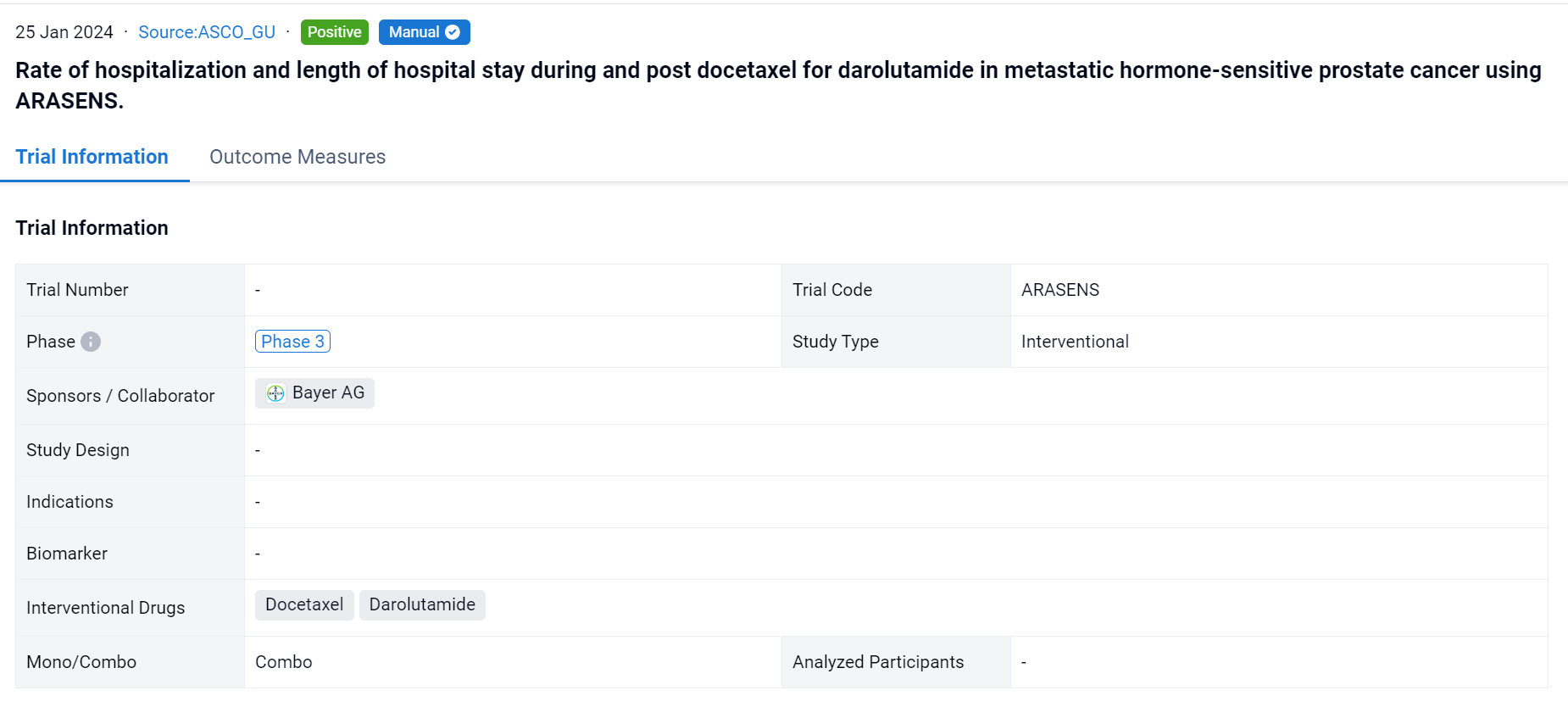
This randomized, double-blind, Phase III trial (NCT05316155) was aimed to compare rates of hospitalizations and length of hospital stay (LoS) per patient due to any reason, and due to adverse events (AE) during or post docetaxel.
In this study, the authors used mixed effects negative binomial regression to estimate time-varying rate of hospitalization and LoS by patient. Patients with records that began on the same start date were considered duplicates and were condensed into one visit, using the latest end date. Patients with multiple records with overlapping time periods were combined into one visit. A constant hospitalization rate assumption was considered inappropriate since docetaxel is administered for 6-cycles (~4.14 months). Hospitalizations that began within and after first 4 months of treatment were grouped together. Conversely, LoS was assumed to be time invariant.
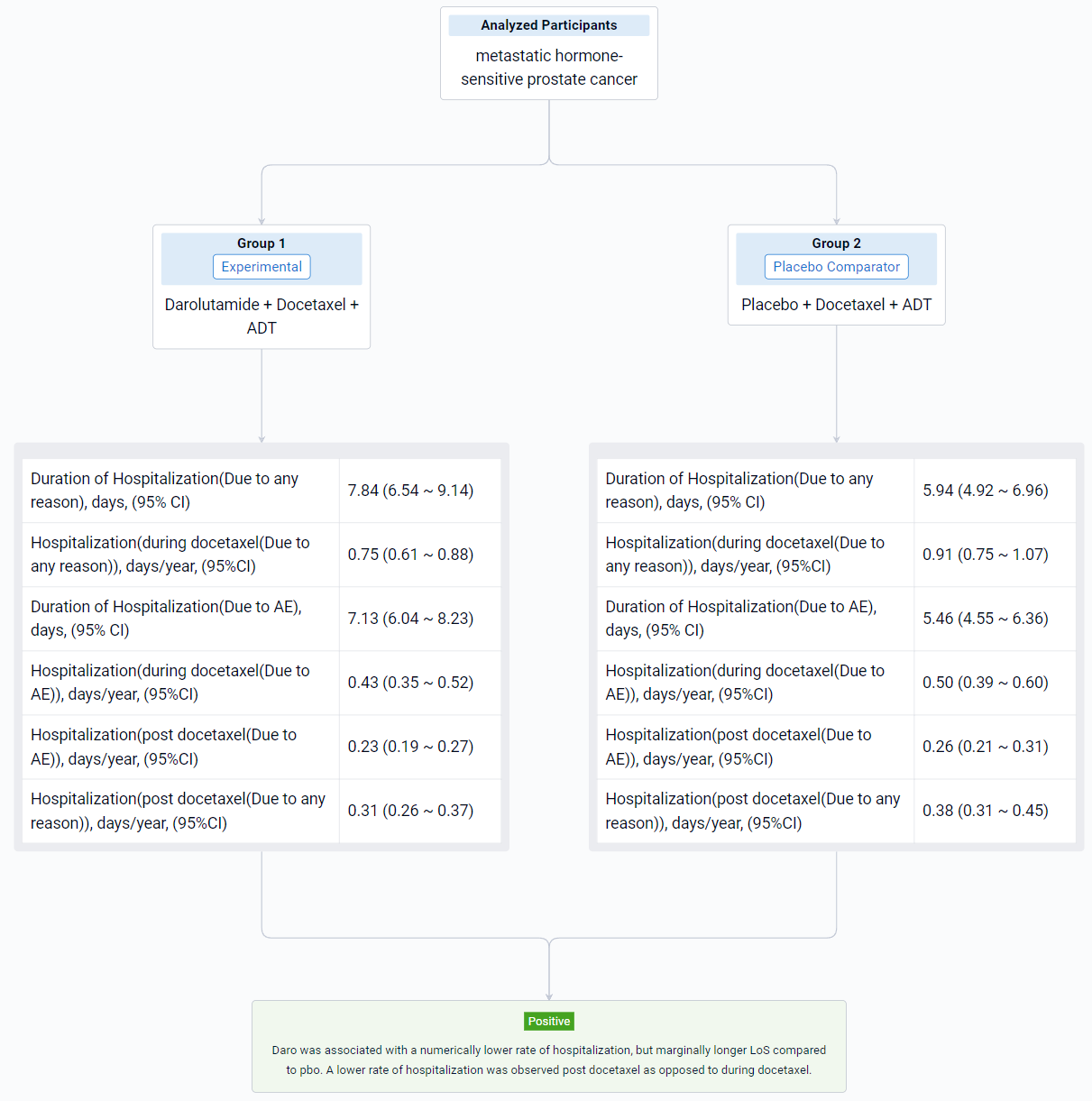
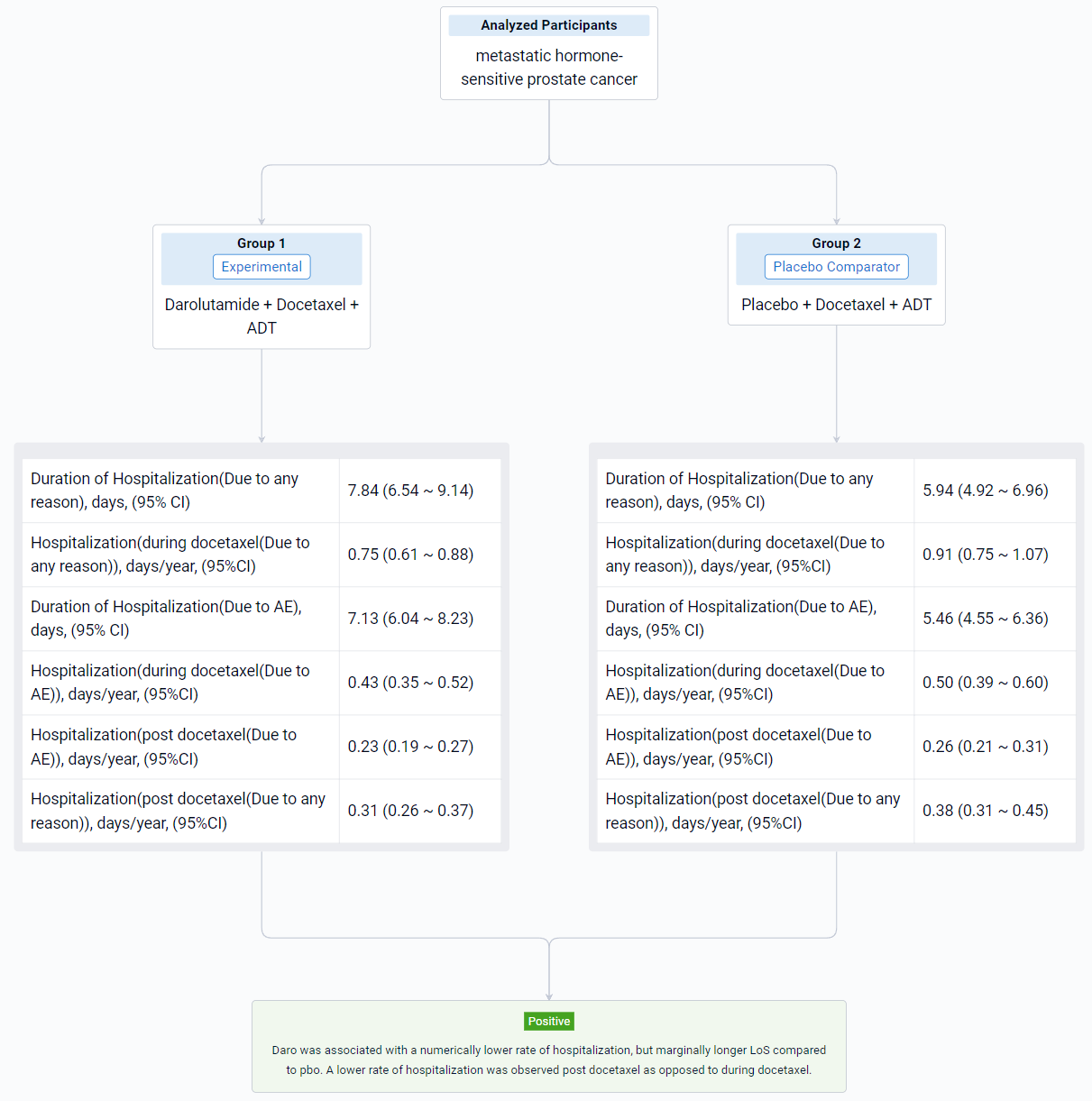
The result showed that the first 4 months of treatment (when patients were receiving docetaxel) were associated with increased hospitalization both due to any reason and to AE (Table 1). Daro was associated with a numerical reduction in rate of hospitalization (per year) due to any reason during docetaxel (0.75 days [95% CI: 0.61, 0.88]) vs. pbo (0.91 days [95% CI: 0.75, 1.07]) and after docetaxel (0.31 days [95% CI: 0.26, 0.37]) vs. pbo (0.38 days [95% CI: 0.31, 0.45]). A similar trend was observed for hospitalizations due to AE. Daro was associated with a marginally longer LoS per hospitalization compared with pbo (+1.90 days). Similar results were seen for LoS due to AE (+1.67 days).
It can be concluded that daro was associated with a numerically lower rate of hospitalization, but marginally longer LoS compared to pbo. A lower rate of hospitalization was observed post docetaxel as opposed to during docetaxel.
How to Easily View the Clinical Results Using Synapse Database?
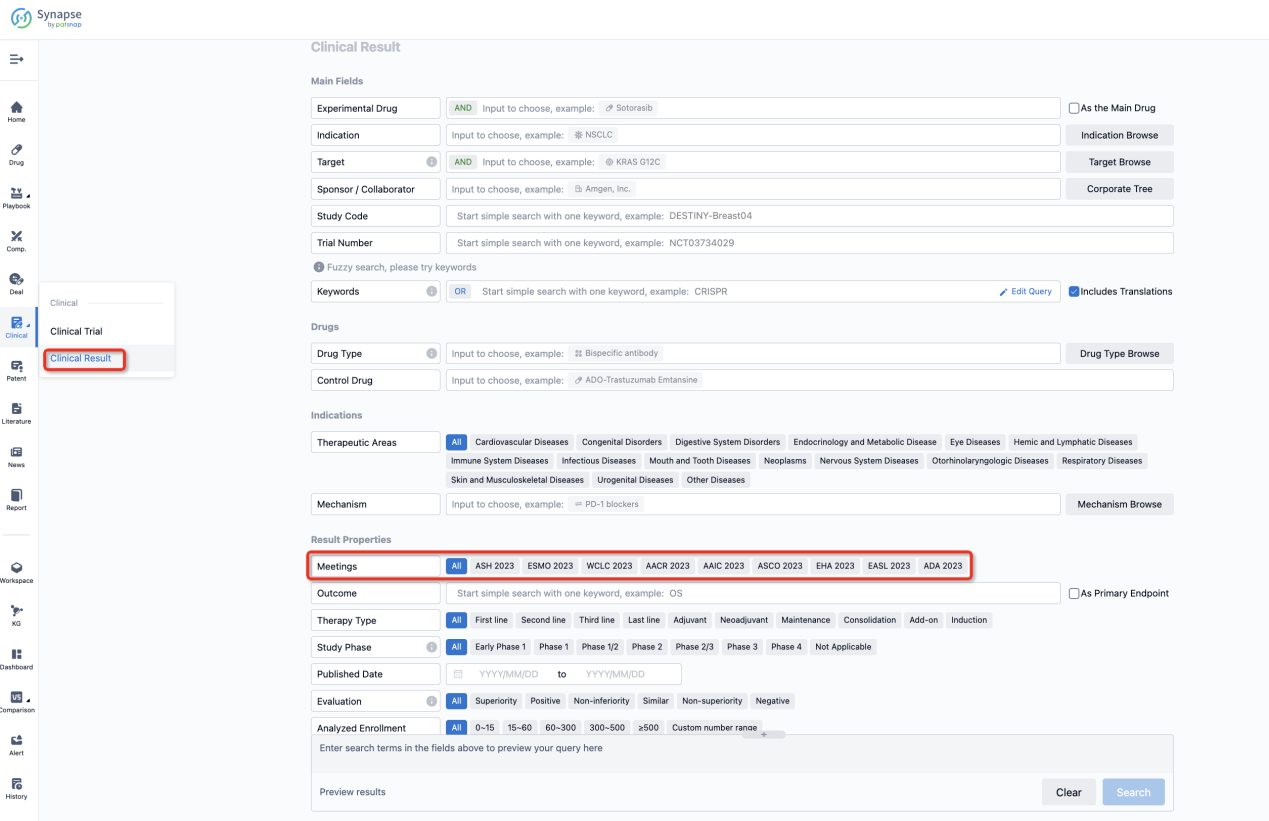
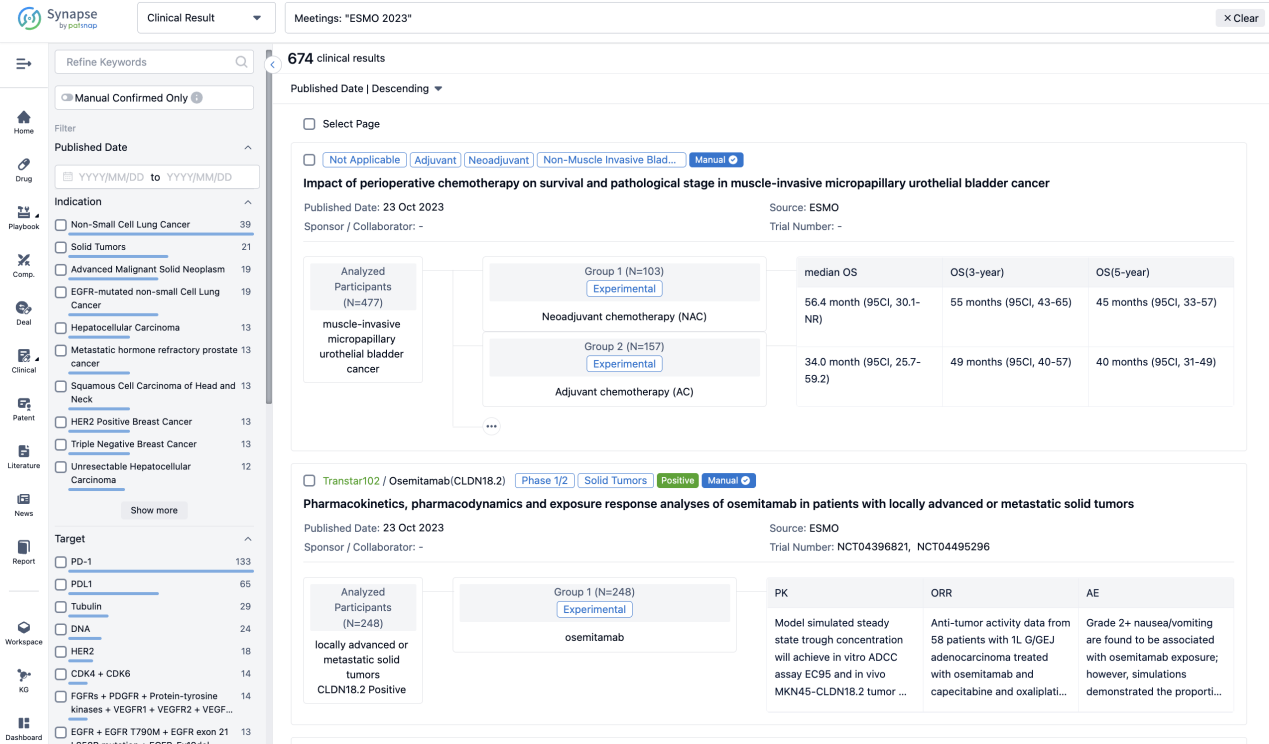
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
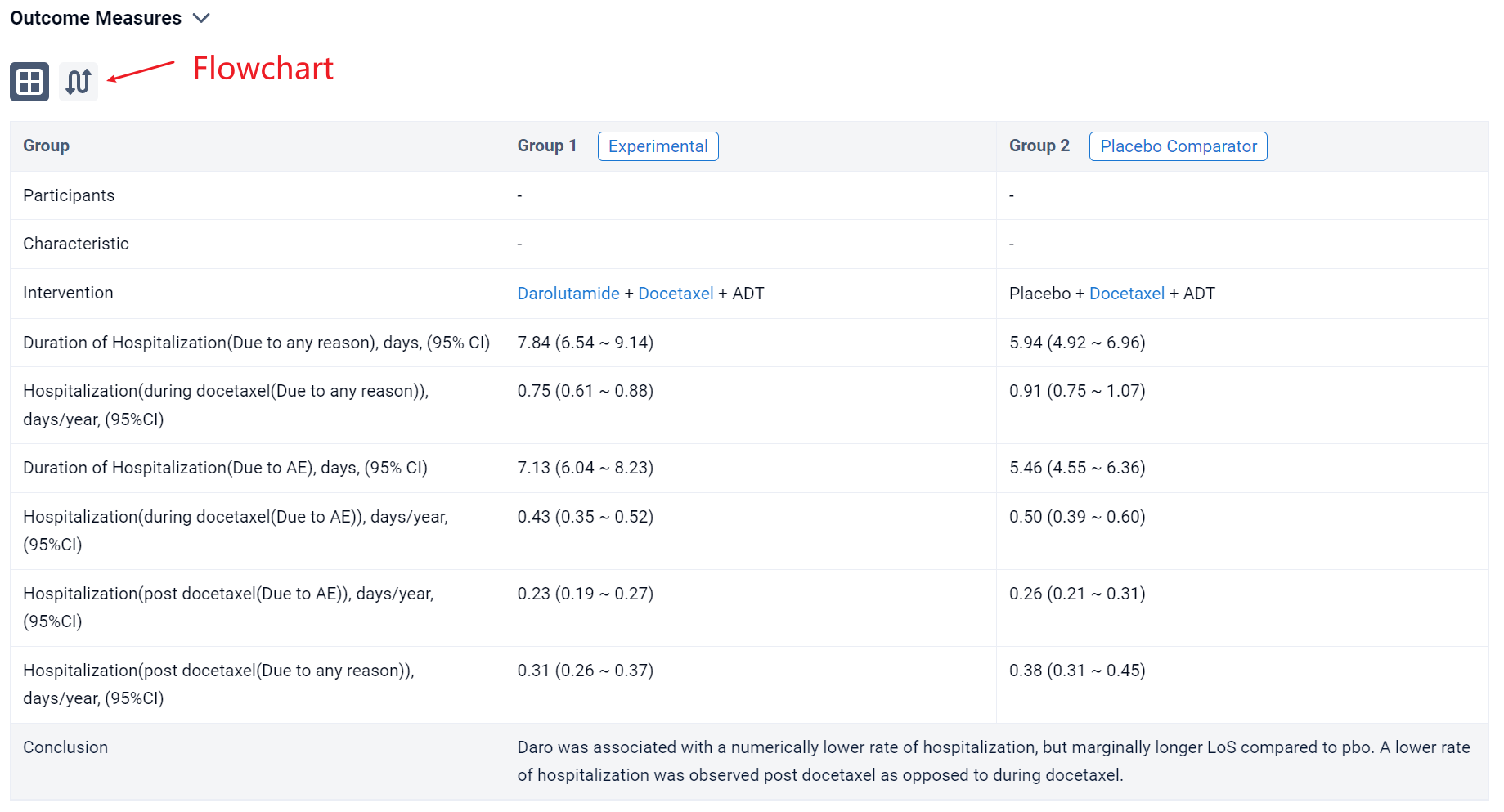
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
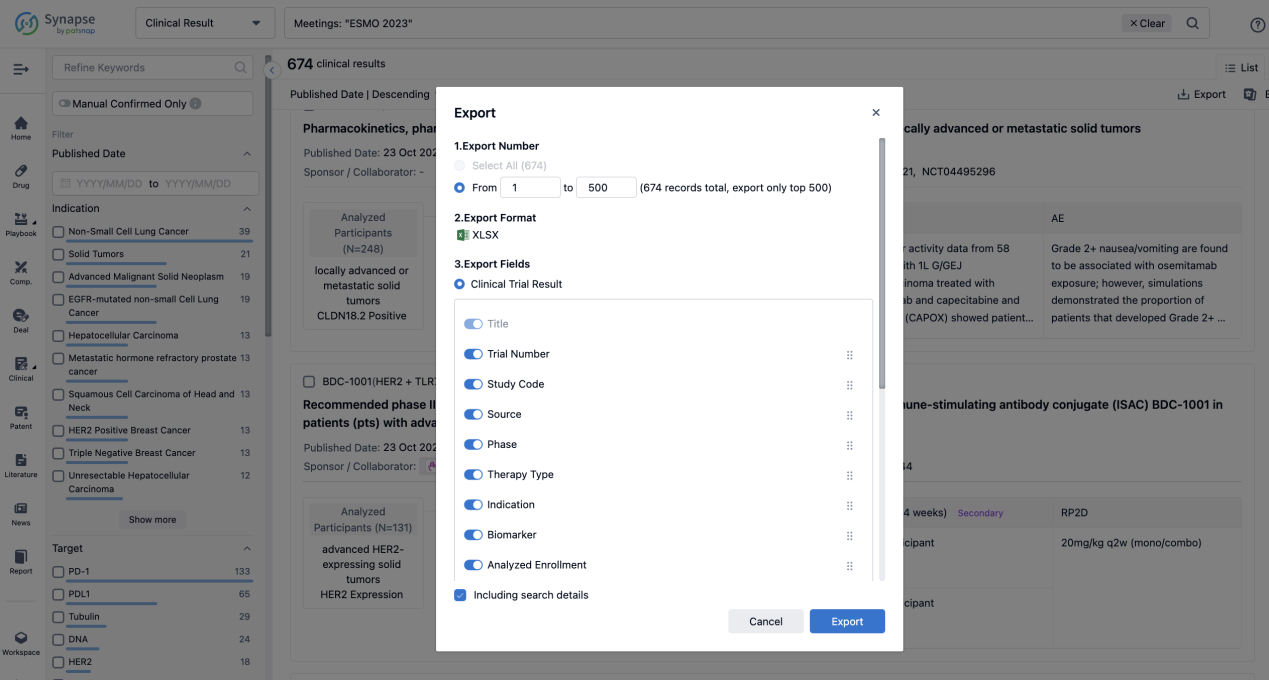
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!