Immune-Onc Therapeutics Announces Partnership for International Early-Stage Trial of IO-108 as First-Line Treatment for Advanced Liver Cancer
Immune-Onc Therapeutics, Inc., has publicized a partnership with Roche for a Phase 1b/2 study, aimed at assessing the efficacy of their pioneering antibody, IO-108, which specifically interacts with LILRB2 (ILT4). This trial will test the combination of IO-108 with atezolizumab and bevacizumab provided by Roche, and it will target individuals diagnosed with either locally advanced, metastatic, or inoperable hepatocellular carcinoma as an initial therapy option.
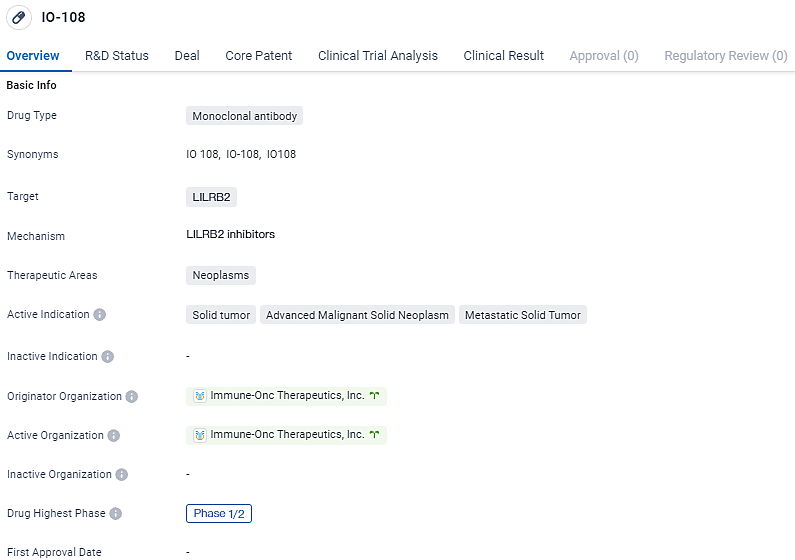
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The combination therapy involving Atezolizumab and Bevacizumab has been recognized by the FDA as an inaugural regimen of immunotherapy for cancer within this particular domain. Moreover, it comes highly recommended by the National Comprehensive Cancer Network as the primary treatment protocol.
Enthusiasm is palpable within Immune-Onc for the partnership with Roche to progress IO-108, as expressed by Charlene Liao, Ph.D., who serves as the CEO and chairperson of the board. According to Dr. Liao, IO-108, both as a single-agent therapy and when used concurrently with T-cell checkpoint blockers, has shown promising therapeutic potential and a tolerable safety margin in a variety of solid cancer types. This strategic alliance signifies a pivotal advance for IO-108, aiming to secure its position as the leading myeloid checkpoint blocker in conjunction with established immunotherapeutic strategies for treating solid malignancies.
With this partnership, Roche takes on the responsibility of sponsoring and orchestrating a comprehensive Phase 1b/2 study on a global scale. This study is designed to appraise the therapeutic impact, safety, and pharmacokinetic properties of IO-108 when used with Atezolizumab and Bevacizumab, in contrast to the established regimen of Atezolizumab and Bevacizumab for advanced hepatic cancer.
Valuable insights from clinical trials in the U.S. evaluating escalating doses of IO-108 were showcased at the AACR Annual Meeting in 2023. The findings revealed that IO-108 was well-tolerated and exhibited positive therapeutic responses as both a standalone therapy and in combination with PD-1 inhibitors across various categories of tumors.
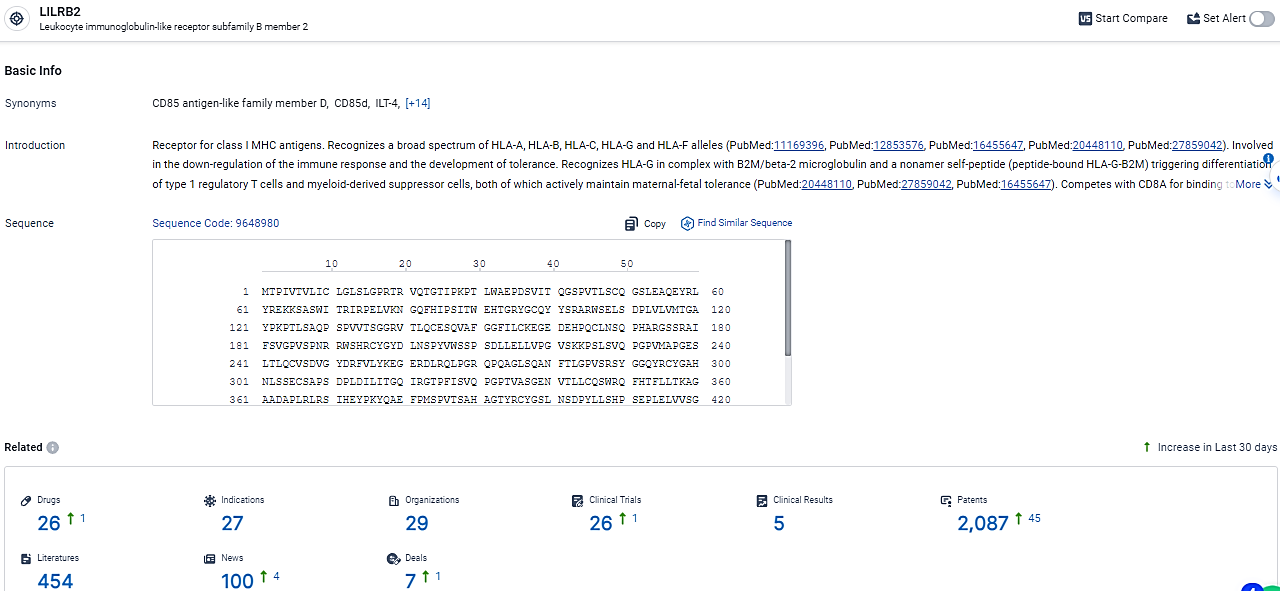
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 26, 2024, there are 26 investigational drugs for the LILRB2 target, including 27 indications,29 R&D institutions involved, with related clinical trials reaching 26, and as many as 2087 patents.
IO-108 is a fully human IgG4 monoclonal antibody with high affinity and specificity towards the myeloid checkpoint, LILRB2. The drug has reached Phase 1/2 trials, both globally and in China, indicating its progress in clinical development. Further research and testing will be necessary to determine the safety and efficacy of IO-108 in treating neoplasms.