Decoding Fosfomycin tromethamine: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Fosfomycin tromethamine's R&D Progress
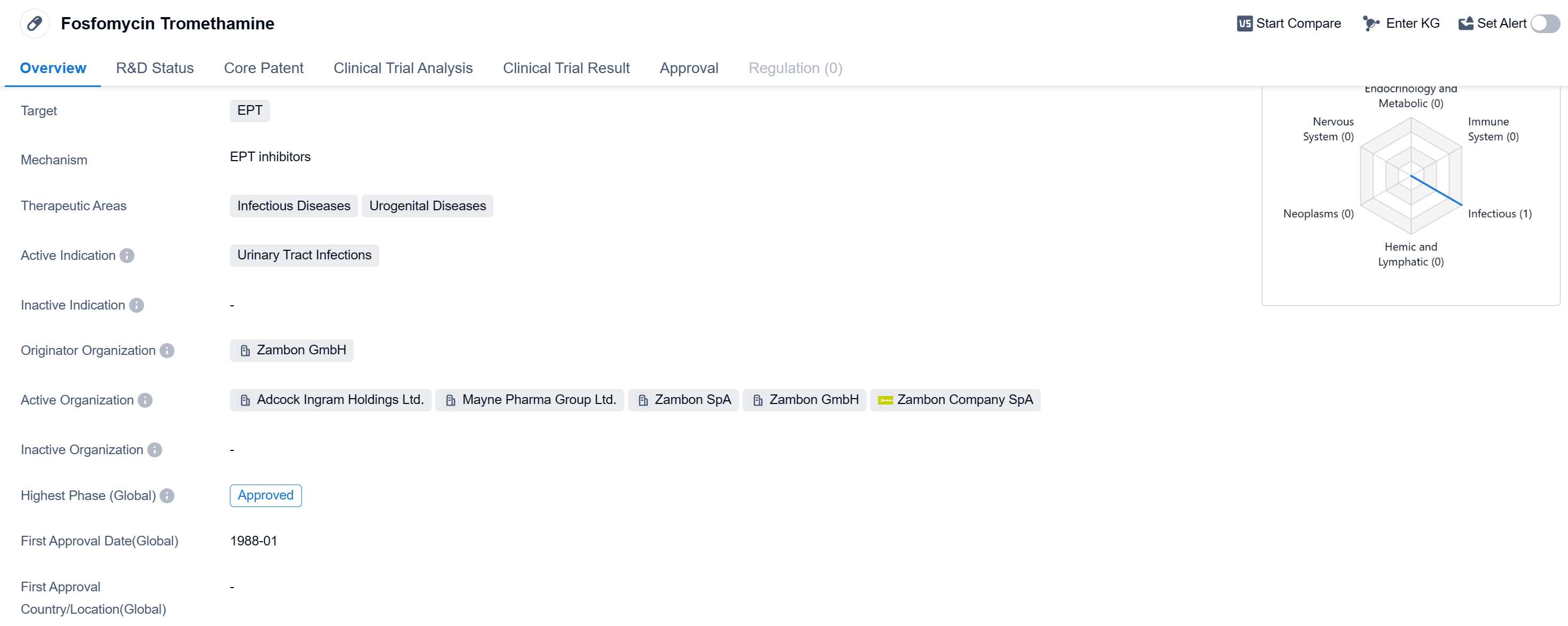
Fosfomycin Tromethamine is a small molecule drug that is primarily used to treat urinary tract infections. It targets the EPT (Escherichia coli, Proteus mirabilis, and Enterococcus faecalis) bacteria, which are commonly associated with these types of infections. The drug falls under the therapeutic areas of infectious diseases and urogenital diseases.
The drug was first approved globally in January 1988, indicating that it has been in use for several decades. It is important to note that the information provided does not specify the specific countries where it has been approved, but it is confirmed that it has received approval globally.
Fosfomycin Tromethamine is developed by Zambon GmbH, an originator organization in the pharmaceutical industry. As an approved drug, it has undergone rigorous testing and evaluation in treating urinary tract infections caused by the targeted bacteria.
Urinary tract infections are a common health issue, particularly among women. Fosfomycin Tromethamine offers a treatment option for patients suffering from these infections, providing relief and potentially preventing complications that may arise if left untreated.
As a small molecule drug, Fosfomycin Tromethamine is likely to have a well-defined chemical structure, making it easier to manufacture and administer. This can contribute to its availability and accessibility for patients in need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Fosfomycin tromethamine: EPT inhibitors
EPT inhibitors are a type of drugs that target and inhibit the activity of the enzyme called EPT (Ethanolaminephosphotransferase). EPT is an enzyme involved in the synthesis of phosphatidylethanolamine (PE), a key component of cell membranes. By inhibiting EPT, EPT inhibitors can reduce the production of PE, which can have various effects on cellular processes.
From a biomedical perspective, EPT inhibitors can be used in the field of biomedicine to study the role of phosphatidylethanolamine in cellular functions and to investigate potential therapeutic applications. They may have implications in the treatment of certain diseases where abnormal phosphatidylethanolamine metabolism is involved, such as cancer or neurodegenerative disorders. Additionally, EPT inhibitors can be valuable tools in understanding the mechanisms underlying cell membrane formation and function.
It is important to note that without further context, it is not possible to provide a more specific explanation or indication for EPT inhibitors.
Drug Target R&D Trends for Fosfomycin tromethamine
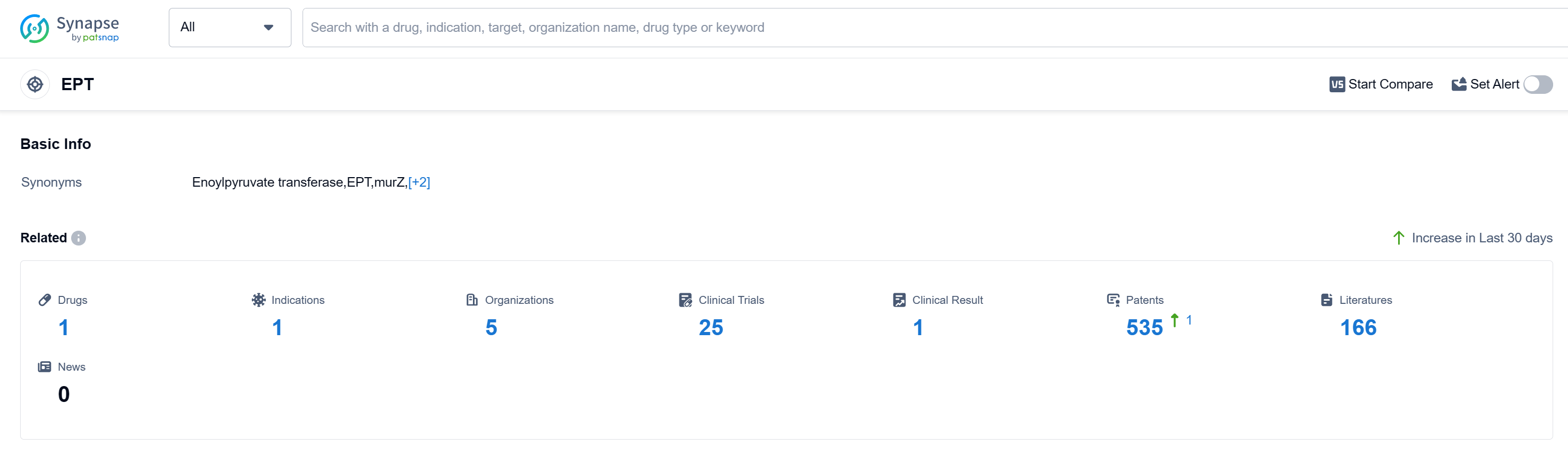
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 1 EPT drugs worldwide, from 5 organizations, covering 1 indications, and conducting 25 clinical trials.
The analysis of the target EPT reveals a competitive landscape with multiple companies successfully developing drugs and obtaining approval. Mayne Pharma Group Ltd., Zambon GmbH, Adcock Ingram Holdings Ltd., and Zambon Company SpA are among the companies that have shown significant growth in this area.
The approved indication for the target EPT is urinary tract infections, indicating successful development in this specific area. Small molecule drugs have shown rapid progress and are the most prominent drug type in the highest development phase.
Various countries/locations have demonstrated progress in the development of drugs under the target EPT, with China being a notable contributor. The presence of multiple countries/locations in the approved stage highlights the global efforts in advancing pharmaceutical research and development for the target EPT.
Overall, the target EPT presents a competitive landscape with diverse companies, drug types, and global participation. The future development of this target holds promise for further advancements in the pharmaceutical industry, particularly in the treatment of urinary tract infections and the utilization of small molecule drugs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Fosfomycin Tromethamine is an approved small molecule drug developed by Zambon GmbH. It targets the EPT bacteria and is primarily used to treat urinary tract infections. With its approval dating back to 1988, it has a long history of use in the field of biomedicine. Its approval in the global markets further highlights its significance in the treatment of urinary tract infections.