Decoding Tasurgratinib: a comprehensive study of its R&D trends and its clinical results in 2024 ASCO_GI
Cholangiocarcinoma (CCA) represents ~3% of all gastrointestinal malignancies globally. Tasurgratinib (E7090) is an orally available selective inhibitor of FGFR 1–3, the recommended dose is 140 mg per day per the dose-escalation part of a first-in-human phase 1 study. And the latest phase 2 clinical data of Tasurgratinib were presented in 2024 ASCO_GI.
Tasurgratinib's R&D Progress
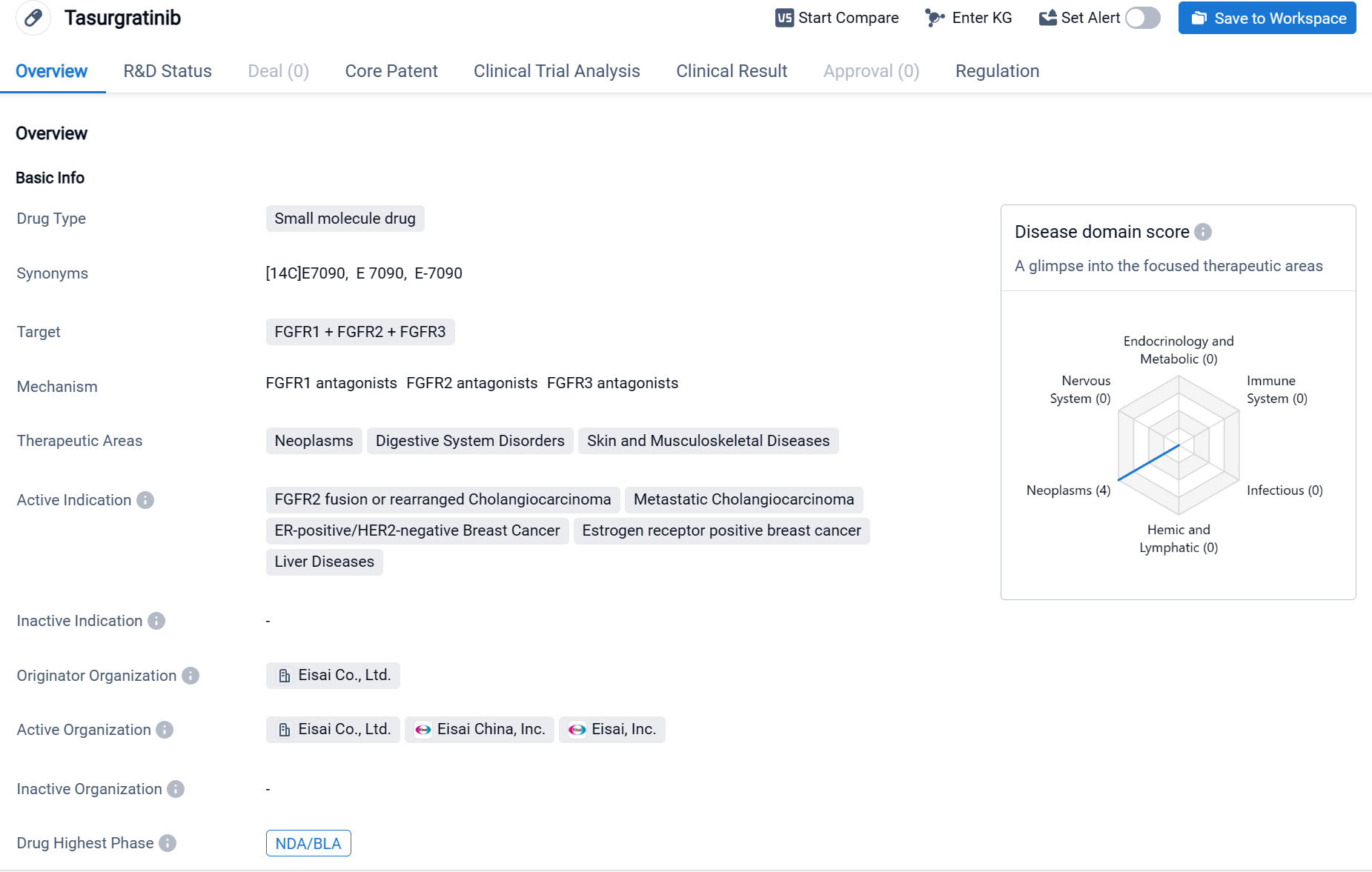
Tasurgratinib is a small molecule drug that falls under the category of biomedicine. It targets three fibroblast growth factor receptors (FGFR1, FGFR2, and FGFR3). The drug is primarily focused on treating neoplasms, digestive system disorders, and skin and musculoskeletal diseases.
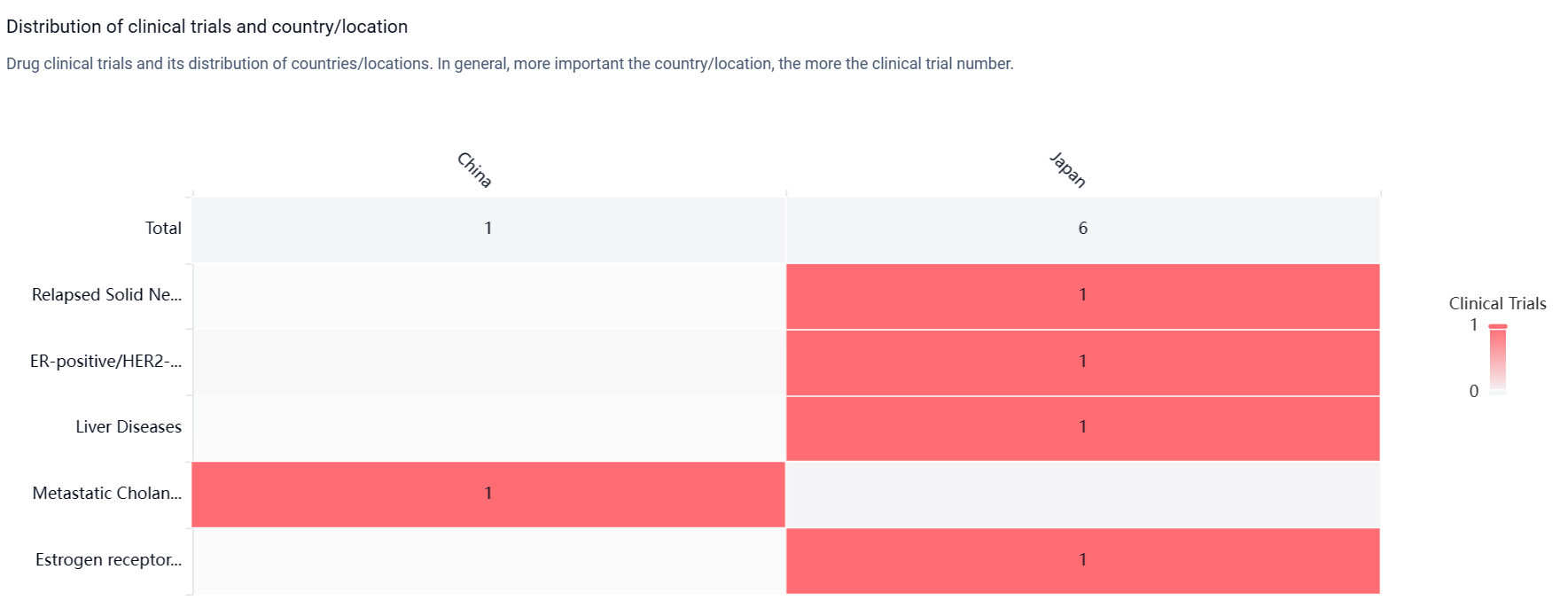
According to the Patsnap Synapse, Tasurgratinib has reached the highest phase of development, which is NDA/BLA globally. In China, it has reached Phase 2 of development. And the clinical trial distributions for Tasurgratinib are primarily in China and Japan. The key indication is Relapsed Solid Neoplasm.
Detailed Clinical Result of Tasurgratinib
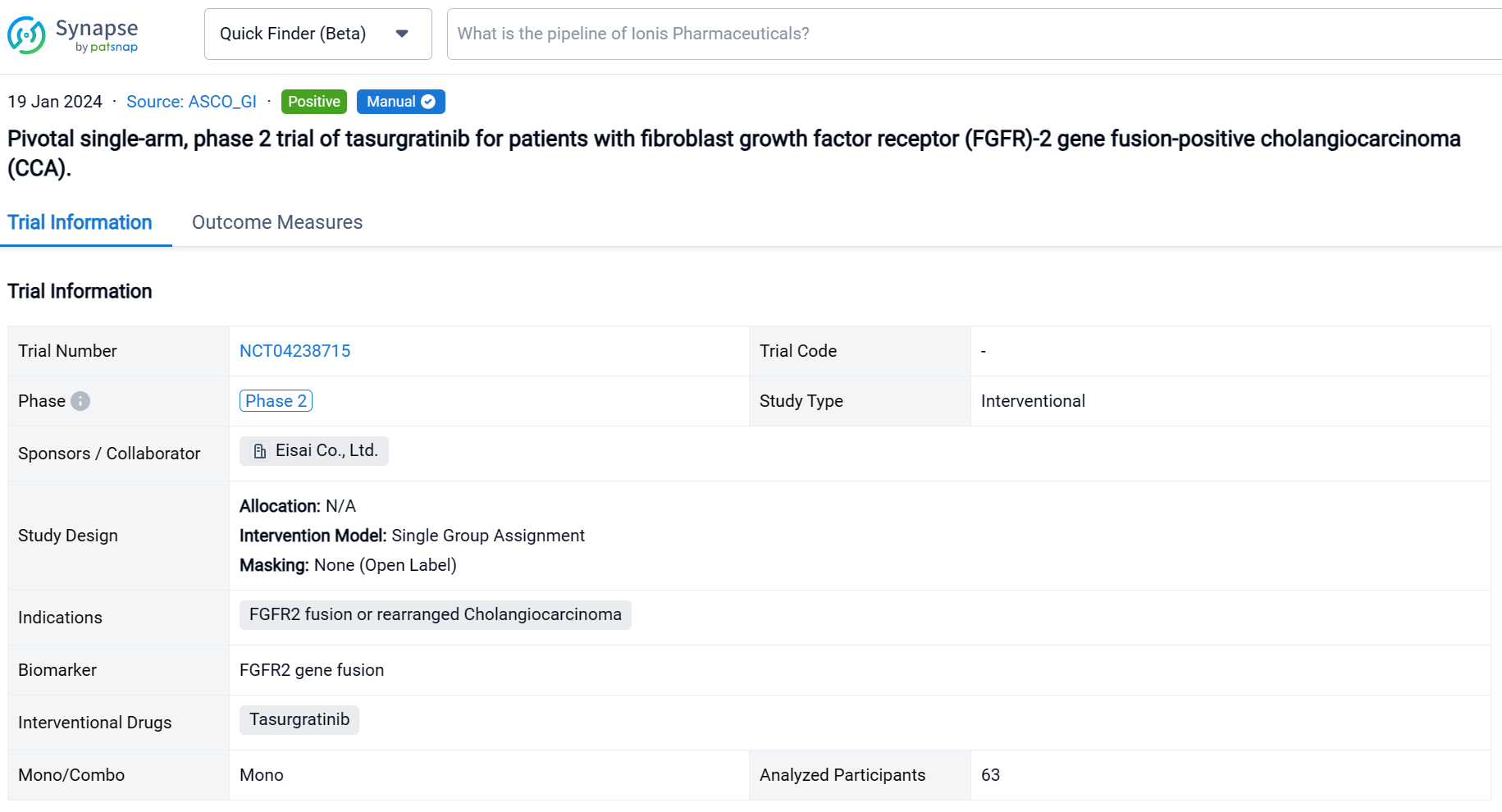
This pivotal single-arm, phase 2 trial (NCT04238715) was aimed to determine the safety and efficacy of Tasurgratinib in patients (pts) with FGFR2 fusion-positive CCA.
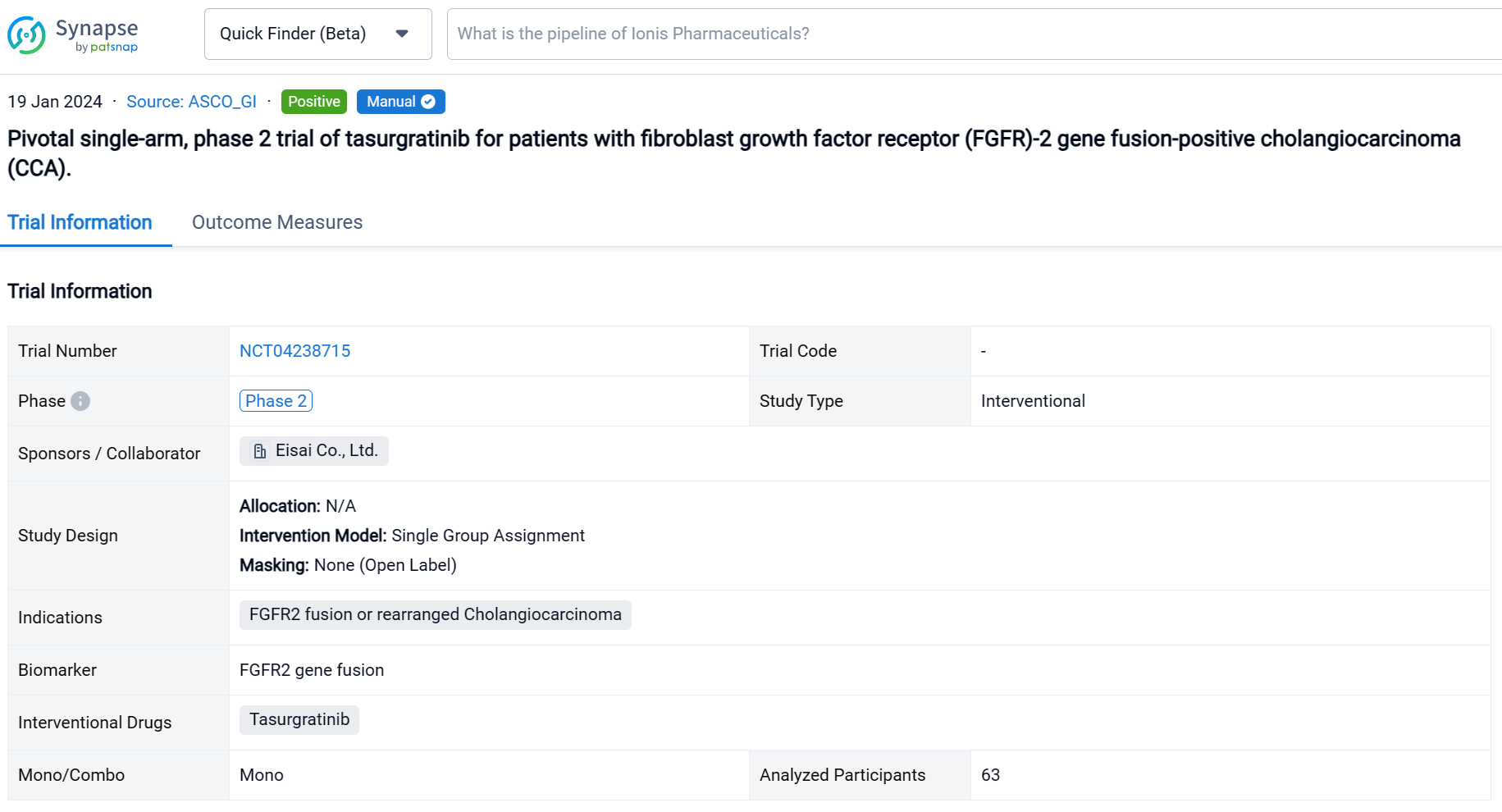
In this study, Japanese and Chinese pts with surgically unresectable advanced or metastatic CCA were treated with tasurgratinib 140 mg PO daily. FGFR2 gene fusion was confirmed by fluorescence in situ hybridization performed in central laboratories; ≥ 1 prior chemotherapy regimen including a gemcitabine-based combination was required; pts treated with FGFR2 inhibitors were excluded. The primary endpoint was objective response rate (ORR; complete response [CR] + partial response [PR]). Secondary endpoints included duration of response (DOR), time to response (TTR), disease control rate (DCR; CR + PR + stable disease [SD]), clinical benefit rate (CBR; CR + PR + SD lasting ≥ 23 weeks), progression-free survival (PFS), overall survival (OS), and safety. The study was considered successful if the lower limit of the ORR 90% CI was > 15% in a planned population. Tumor responses were measured every 8 weeks by RECIST v1.1 per independent imaging review. Adverse event (AE) severity was measured per CTCAE v4.03.
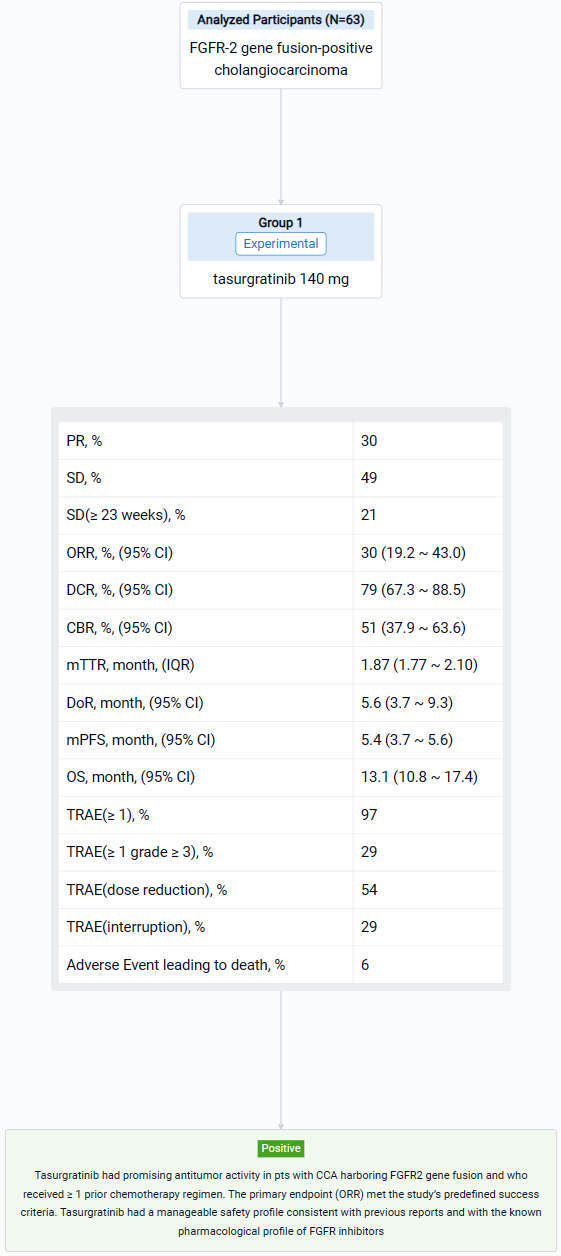
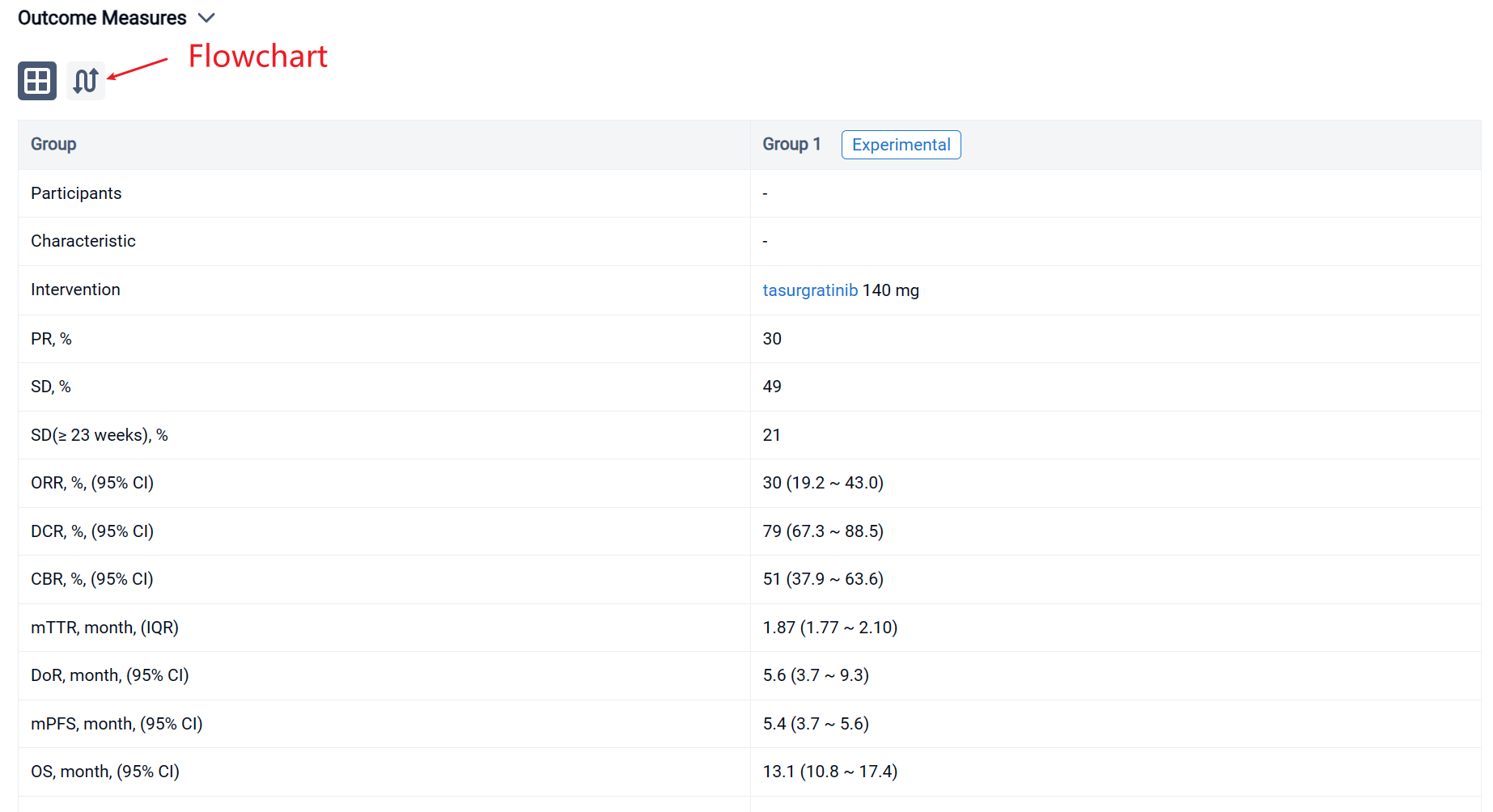
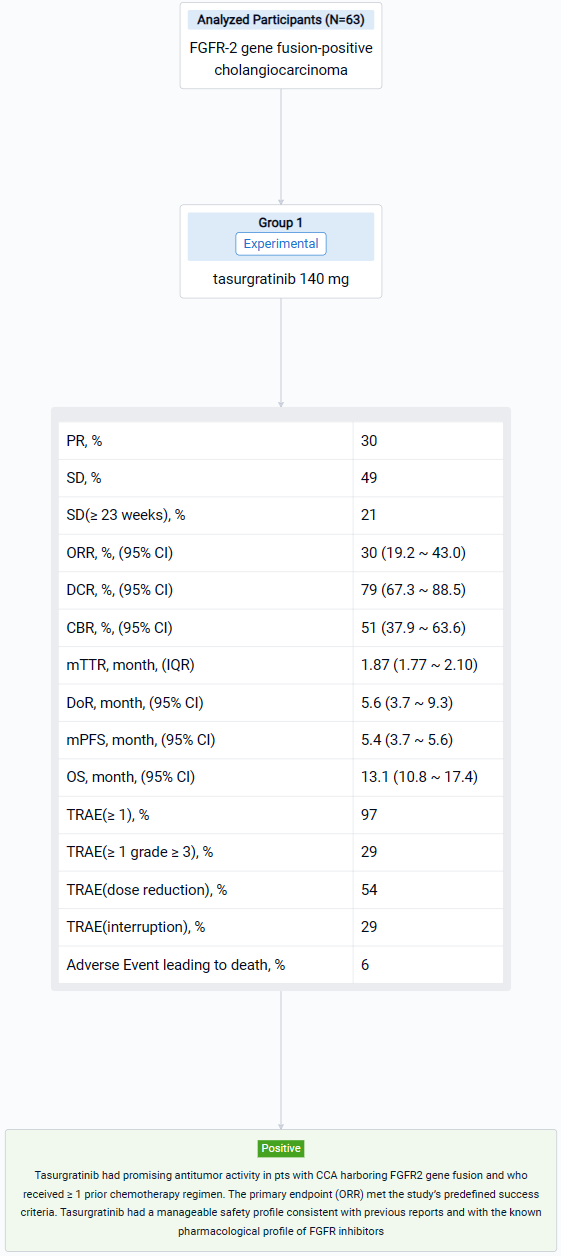
The result showed that 63 Pts (Japanese: n = 28; Chinese: n = 35) were treated (median age: 55 years); 23 pts (37%) had received 1 prior regimen, all others had received ≥ 2. By the data cutoff date (March 15, 2023), 55 pts discontinued treatment (disease progression, n = 48; adverse events, n = 4; pt choice, n = 2; loss of clinical benefit, n = 1). 19 Pts (30%) had a PR; 31 pts (49%) had SD and 13 (21%) had SD for ≥ 23 weeks. The ORR was 30% (2-sided 90% CI 20.7–41.0; 95% CI 19.2–43.0); the DCR was 79% (95% CI 67.3–88.5); the CBR was 51% (95% CI 37.9–63.6). The median [m]TTR / DOR for responders were 1.87 mos (IQR 1.77–2.10; range 1.6–14.7) / 5.6 mos (95% CI 3.7–9.3; range 1.0+–14.8+). The mPFS / OS were 5.4 mos (95% CI 3.7–5.6) / 13.1 mos (95% CI 10.8–17.4). 61 Pts (97%) had ≥ 1 treatment-related AE (TRAE), most commonly hyperphosphatemia (n = 51; 81%); 18 pts (29%) had ≥ 1 grade ≥ 3 TRAE, most commonly lipase increased (n = 4; 6%). 34 Pts (54%) had a dose reduction and 18 (29%) had treatment interruption due to TRAEs. 4 Pts (6%) had a fatal AE, none related to treatment.
It can be concluded that Tasurgratinib had promising antitumor activity in pts with CCA harboring FGFR2 gene fusion and who received ≥ 1 prior chemotherapy regimen. The primary endpoint (ORR) met the study’s predefined success criteria. Tasurgratinib had a manageable safety profile consistent with previous reports and with the known pharmacological profile of FGFR inhibitors.
How to Easily View the Clinical Results Using Synapse Database?
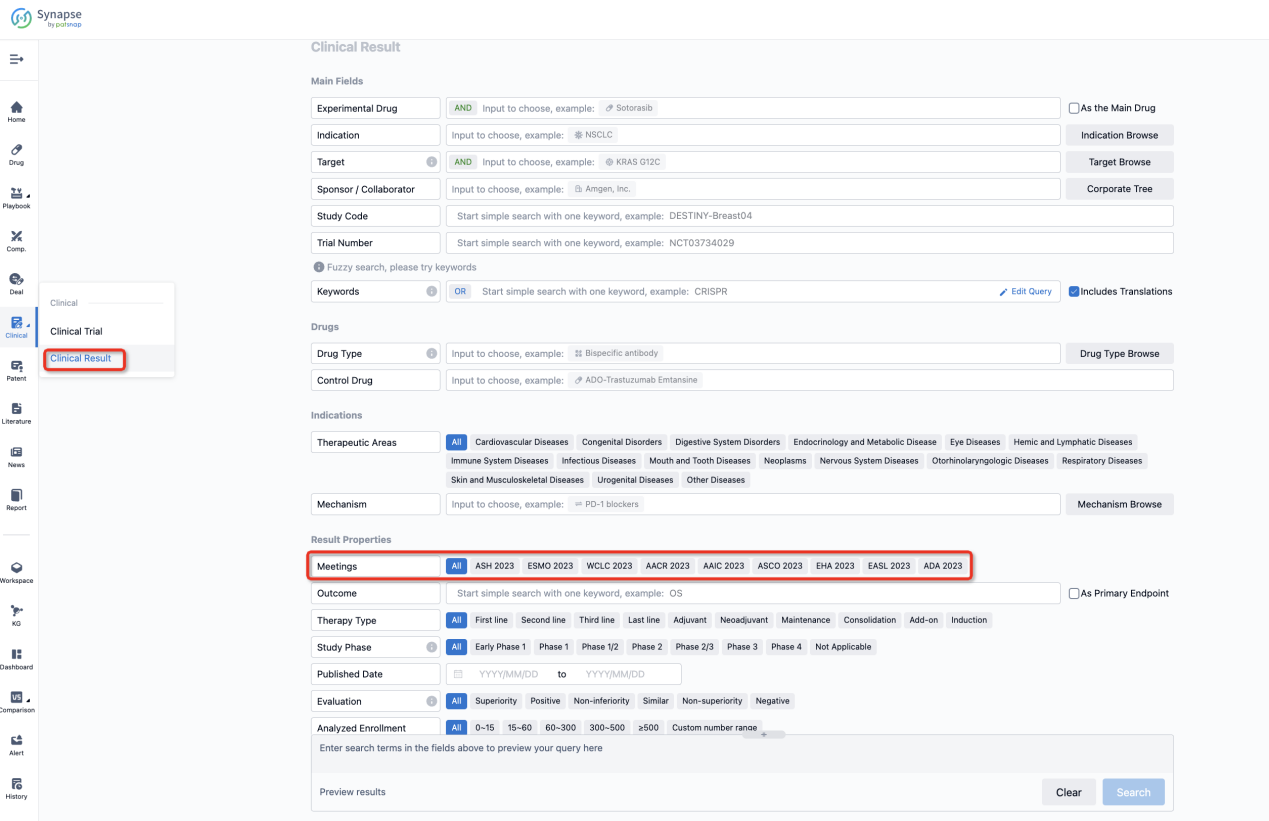
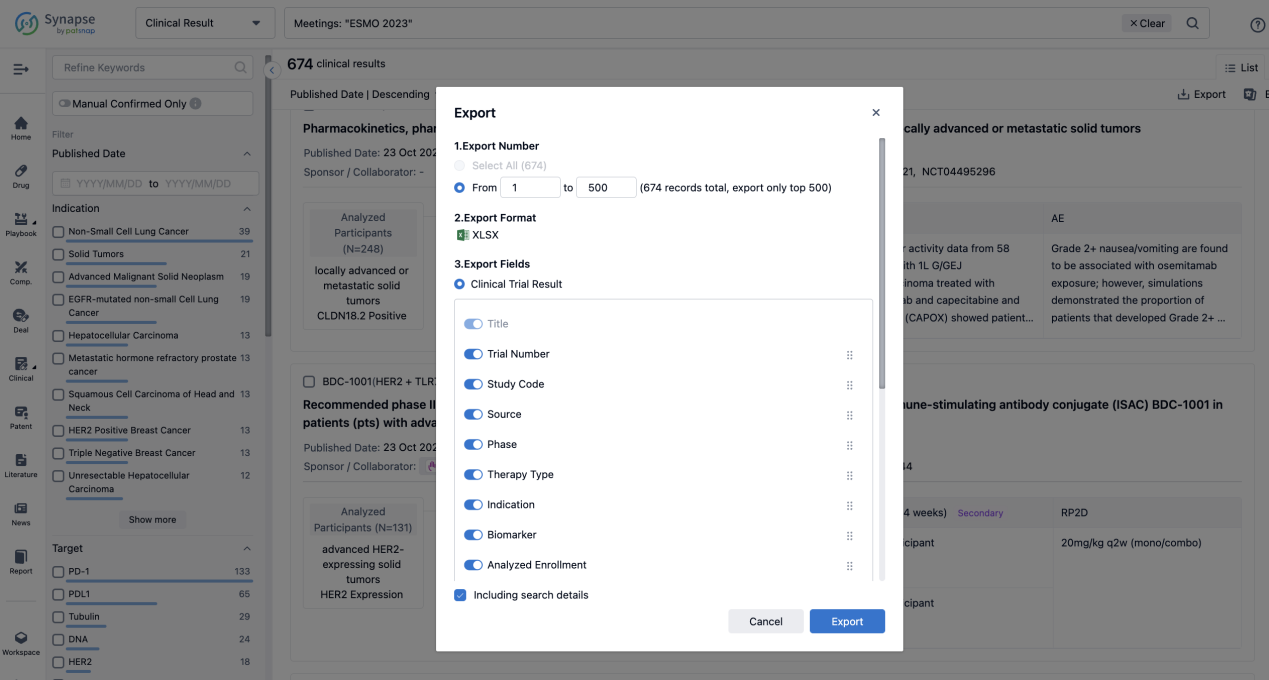
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
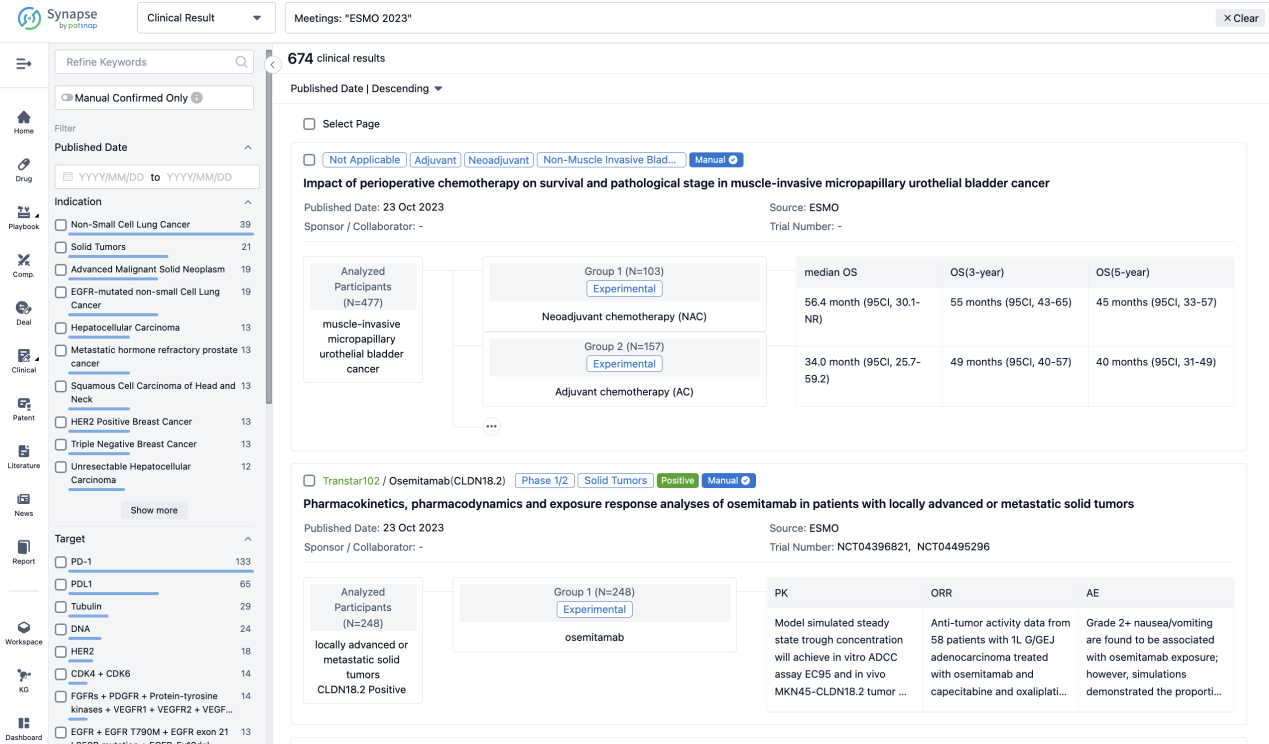
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!