Hemab Therapeutics reveals promising early results for Glanzmann's Thrombasthenia drug, HMB-001, at 2024 EAHAD Congress
Hemab Therapeutics, an emergent biotech firm in the clinical phase focused on creating new preventative treatments for severe and inadequately addressed hematological conditions related to bleeding and thrombosis, has shared preliminary findings from the first phase of its continual assessment of HMB-001.
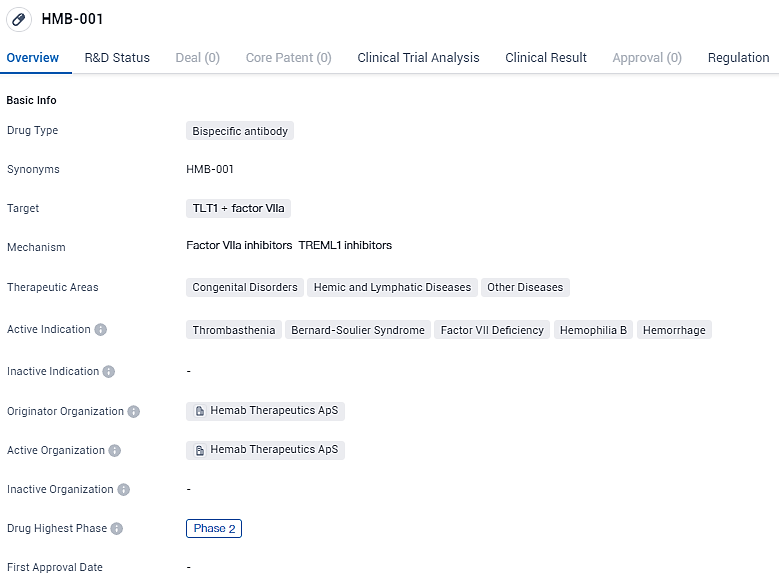
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
A pioneering bispecific antibody, coined HMB-001, is currently under development as a preventive approach for managing Glanzmann Thrombasthenia, a bleeding condition. Highlights of this research were among the 10 chosen abstracts presented at the SLAM session during the 17th Annual European Association for Haemophilia and Allied Disorders Congress in Frankfurt, Germany, this week.
“Initial clinical findings for HMB-001 in managing Glanzmann indicate that the underlying method displays promise as a novel preventive solution for individuals grappling with underrecognized coagulopathies, who endure daily risks of severe and possibly fatal hemorrhages,” commented Joe Vogel, Senior Director and Project Lead for HMB-001 at Hemab.
Administering HMB-001 has led to a tiered pharmacodynamic response marked by the accrual of endogenous Factor VIIa, correlated with reductions in prothrombin time and enhancements noted in preliminary thrombin generation studies. The pharmacokinetic characteristics and persistence in the bloodstream of HMB-001 allow for biweekly administration or perhaps a more extended interval between doses.
“Individuals contending with Glanzmann Thrombasthenia face traumatizing hemorrhages and significant psychological distress, ranging from acute anguish and societal shaming to depressive episodes and solitude, yet lack sanctioned proactive treatment options,” noted Suthesh Sivapalaratnam, MD, PhD, of Queen Mary University of London and Barts Health NHS Trust. “The initial phase of clinical research corroborates the need for continued exploration into the potential of HMB-001 to elevate care standards for patients and health professionals globally who are in search of advancements.”
Moreover, Hemab has broadcasted that the preclinical insights on HMB-001 are now accessible within the online February 2024 edition of Nature Cardiovascular Research. Research outcomes underline the proficiency of HMB-001 in enhancing and extending the action of innate Factor VIIa selectively on active platelets, facilitating a prolonged, focused prohemostatic effect which could be integral in the preventive therapy for Glanzmann Thrombasthenia or other hemophilia conditions. The full article is available for review here.
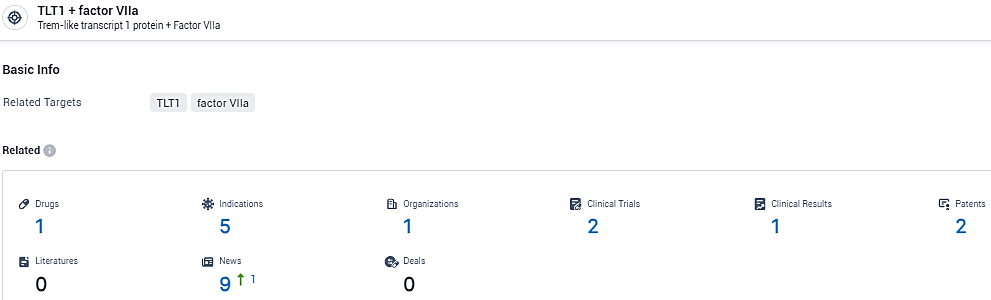
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 19, 2024, there are 1 investigational drugs for the TLT1 and factor VIIa target, including 5 indications, 1 R&D institutions involved, with related clinical trials reaching 2, and as many as 2 patents.
HMB-001 targets TLT1 and factor VIIa and has shown potential in treating various congenital disorders, hemic and lymphatic diseases, and other conditions. With its Fast Track designation, HMB-001 has the potential to address unmet medical needs and provide a new treatment option for patients suffering from these conditions.