Deep Scientific Insights on Dabigatran Etexilate Mesylate's R&D Progress
Dabigatran Etexilate Mesylate's R&D Progress
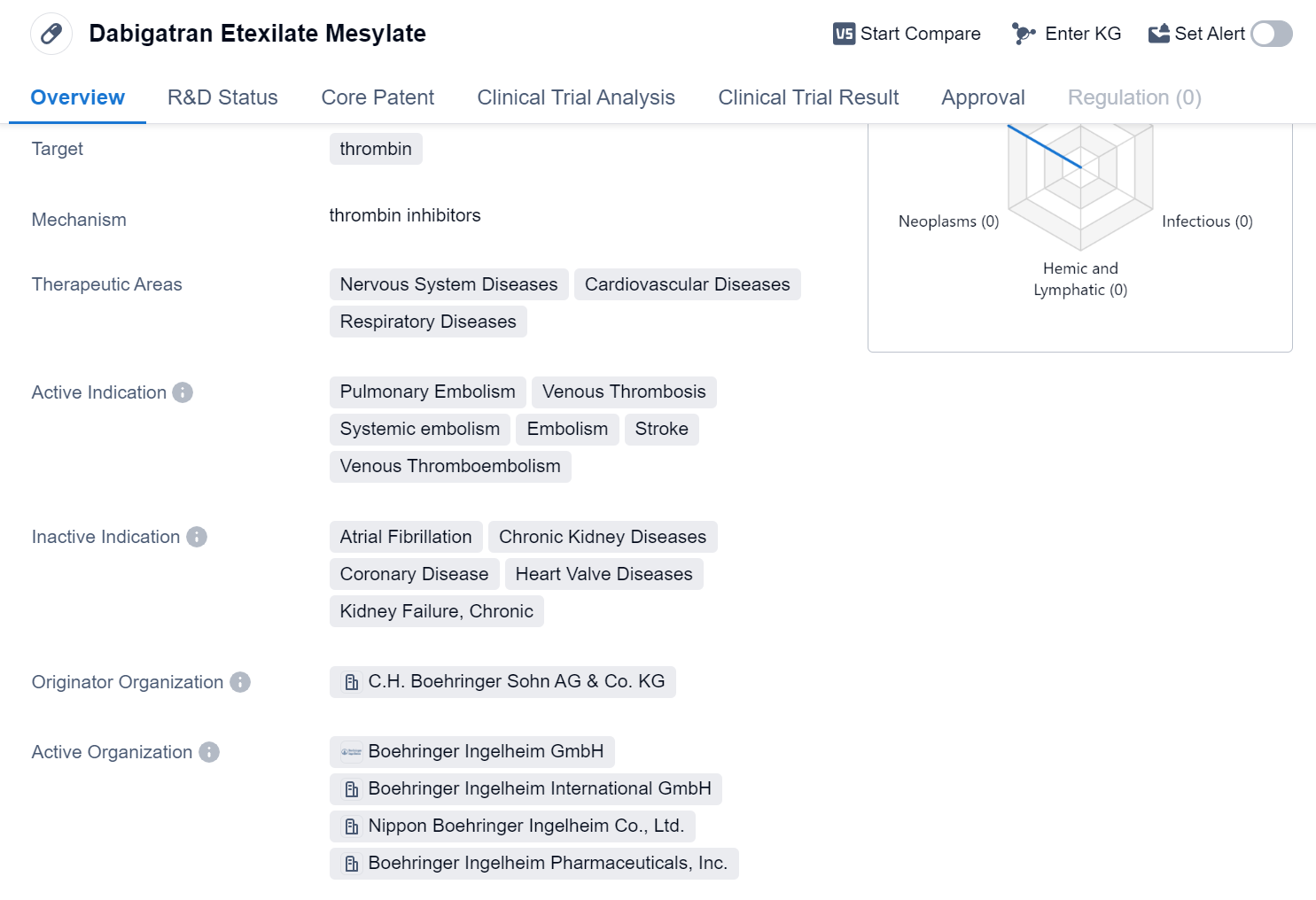
Dabigatran Etexilate Mesylate is a small molecule drug that targets thrombin, an enzyme involved in blood clotting. It has been approved for use in the treatment of various conditions related to the nervous system, cardiovascular system, and respiratory system. The drug is primarily indicated for the treatment of pulmonary embolism, venous thrombosis, systemic embolism, embolism, stroke, and venous thromboembolism.
The originator organization of Dabigatran Etexilate Mesylate is C.H. Boehringer Sohn AG & Co. KG. The drug has received approval in multiple countries, with its first approval granted in the European Union in March 2008. It is important to note that the highest phase of development for this drug is approved, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization.
Dabigatran Etexilate Mesylate is a significant advancement in the field of biomedicine, particularly in the treatment of thrombotic disorders. By targeting thrombin, the drug helps prevent the formation of blood clots, which can lead to serious complications such as pulmonary embolism and stroke. Its approval in multiple therapeutic areas suggests its potential efficacy in addressing a range of diseases affecting the nervous, cardiovascular, and respiratory systems.
The drug's approval in the global market highlights its widespread acceptance and recognition by regulatory authorities.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Dabigatran Etexilate Mesylate: Thrombin inhibitors
Thrombin inhibitors are a type of medication that specifically target and inhibit the activity of thrombin, which is a key enzyme involved in blood clotting. Thrombin plays a crucial role in the formation of blood clots by converting fibrinogen into fibrin, a protein that forms the structural framework of a clot. By inhibiting thrombin, these medications help prevent the formation of blood clots or dissolve existing clots.
From a biomedical perspective, thrombin inhibitors are commonly used in the treatment and prevention of various thrombotic disorders, such as deep vein thrombosis, pulmonary embolism, and stroke. They can also be used during certain medical procedures, such as cardiac surgeries or angioplasty, to reduce the risk of clot formation. Thrombin inhibitors work by interfering with the coagulation cascade, which is a series of complex reactions that ultimately lead to clot formation. By blocking thrombin, these medications disrupt the cascade and prevent the formation of stable blood clots.
There are different types of thrombin inhibitors, including direct thrombin inhibitors and indirect thrombin inhibitors. Direct thrombin inhibitors directly bind to thrombin and inhibit its activity, while indirect thrombin inhibitors target other molecules in the coagulation cascade to indirectly inhibit thrombin. Examples of direct thrombin inhibitors include medications like dabigatran and argatroban, while indirect thrombin inhibitors include heparin and warfarin.
Thrombin inhibitors are an essential component of anticoagulant therapy and are prescribed based on the specific condition being treated, the patient's medical history, and other individual factors. It is important to carefully monitor patients receiving thrombin inhibitors to ensure appropriate dosing and to minimize the risk of bleeding complications, which can be a potential side effect of these medications.
Drug Target R&D Trends for Dabigatran Etexilate Mesylate
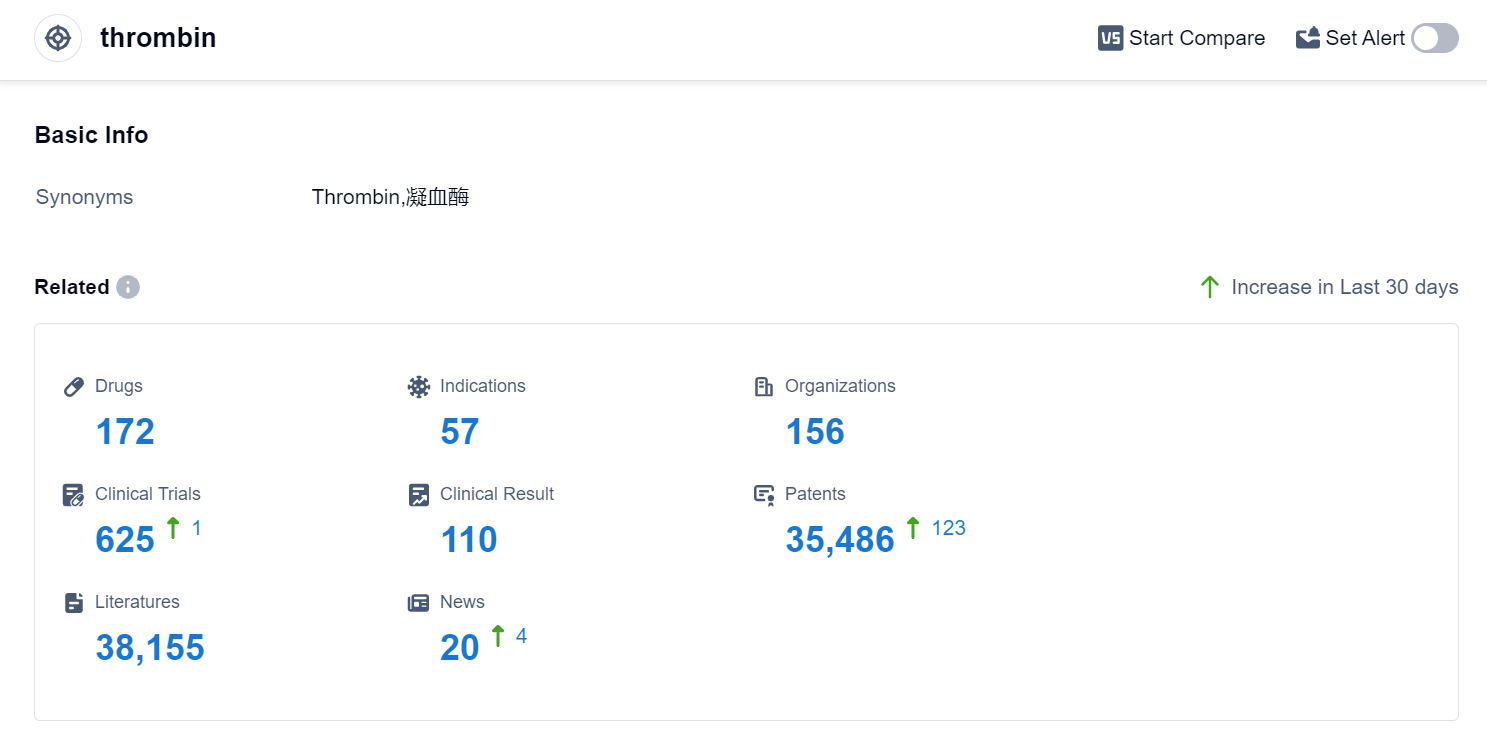
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 172 thrombin drugs worldwide, from 156 organizations, covering 57 indications, and conducting 625 clinical trials.
The analysis of the current competitive landscape and future development of target thrombin highlights the key players, indications, drug types, and countries/locations involved. Grifols SA, Hualan Biological Engineering, Inc., and Shaanxi Coal & Chemical Industry Group Co., Ltd. are the companies with the highest stage of development. Hemorrhage, hemophilia B, thrombosis, and venous thromboembolism are the most common indications. Non-recombinant coagulation factors, small molecule drugs, and blood components are the most rapidly progressing drug types. China, the United States, and the European Union are the leading countries/locations in the development of drugs targeting thrombin. The future development of target thrombin holds promising opportunities for innovative therapies in managing bleeding disorders and preventing blood clots.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Dabigatran Etexilate Mesylate is a small molecule drug that targets thrombin and has been approved for the treatment of various conditions related to the nervous, cardiovascular, and respiratory systems. Its approval in multiple therapeutic areas and countries underscores its potential as a valuable treatment option for patients suffering from thrombotic disorders.