Deep Scientific Insights on Nalbuphine Hydrochloride's R&D Progress
Nalbuphine Hydrochloride's R&D Progress
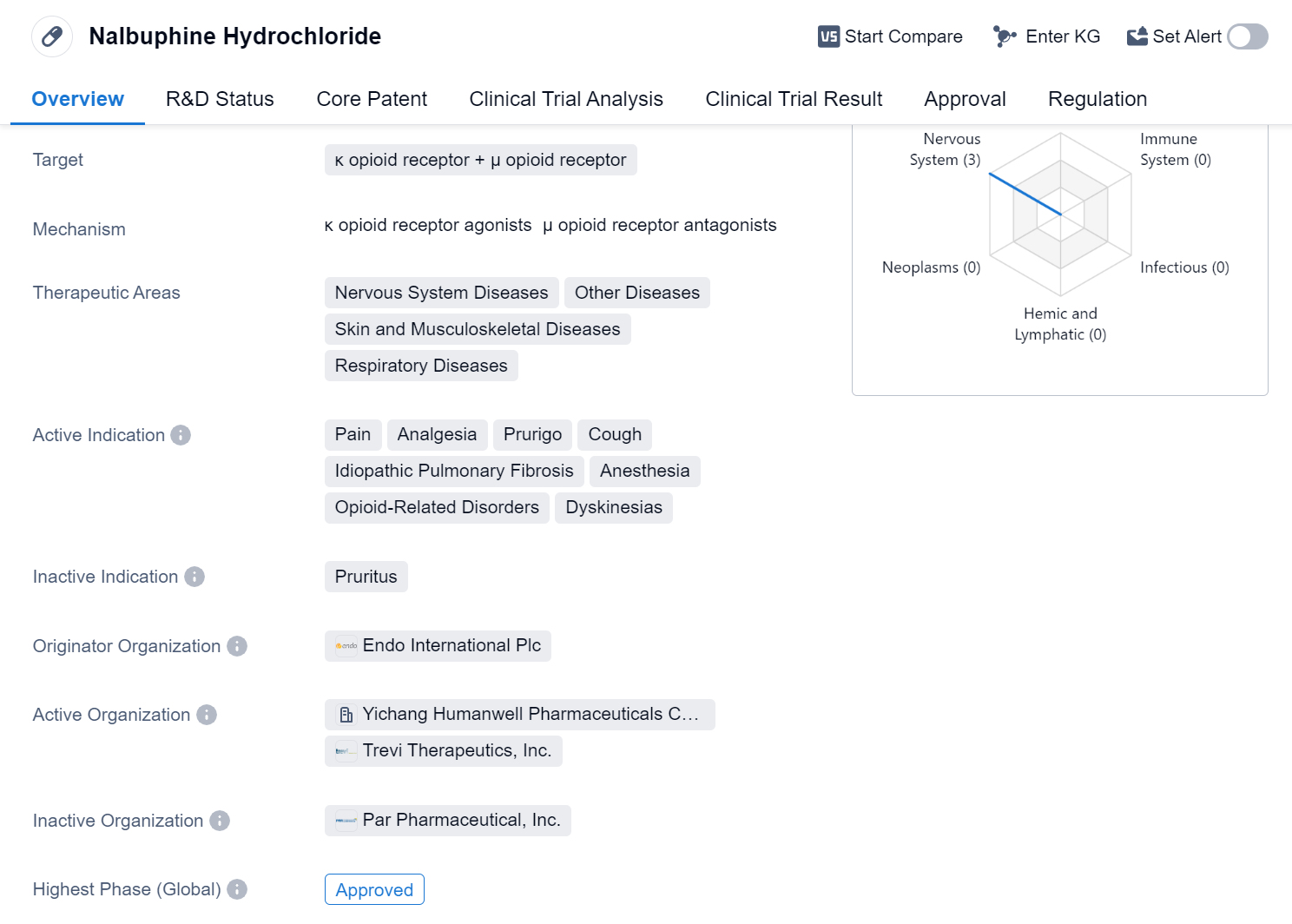
Nalbuphine Hydrochloride is a small molecule drug that targets both the κ opioid receptor and μ opioid receptor. It is primarily used in the treatment of various nervous system diseases, other diseases, skin and musculoskeletal diseases, and respiratory diseases. The drug has multiple active indications, including pain, analgesia, prurigo, cough, idiopathic pulmonary fibrosis, anesthesia, opioid-related disorders, and dyskinesias.
The originator organization of Nalbuphine Hydrochloride is Endo International Plc. The drug has reached the highest phase of approval globally. It received its first approval in the United States in May 1979. Nalbuphine Hydrochloride is regulated under the Fast Track program, which expedites the development and review process of drugs that address unmet medical needs.
As a small molecule drug, Nalbuphine Hydrochloride is designed to interact with specific receptors in the body, namely the κ opioid receptor and μ opioid receptor. By targeting these receptors, the drug aims to provide therapeutic benefits in the form of pain relief, analgesia, and treatment of various diseases affecting the nervous system, skin, musculoskeletal system, and respiratory system.
The approval of Nalbuphine Hydrochloride in multiple therapeutic areas highlights its versatility and potential to address a wide range of medical conditions. Its active indications cover pain management, including acute and chronic pain, as well as the treatment of prurigo, a condition characterized by intense itching. The drug also shows promise in managing cough, idiopathic pulmonary fibrosis, anesthesia, opioid-related disorders, and dyskinesias.
With its first approval dating back to 1979, Nalbuphine Hydrochloride has a long history of use in the United States. Its approval in China further expands its reach and potential patient population. The Fast Track designation indicates that the drug has demonstrated promising results in early clinical trials, and its expedited regulatory pathway reflects the urgent need for effective treatments in the indicated therapeutic areas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nalbuphine Hydrochloride: κ opioid receptor agonists & μ opioid receptor antagonists
κ opioid receptor agonists and μ opioid receptor antagonists are two types of drugs that interact with specific receptors in the brain and nervous system.
From a biomedical perspective, the κ opioid receptor agonists refer to a class of drugs that bind to and activate the κ opioid receptors. These receptors are primarily found in the brain and spinal cord and play a role in modulating pain perception, mood, and stress response. By activating the κ opioid receptors, these agonists can produce analgesic (pain-relieving) effects and may also have sedative and hallucinogenic properties.
On the other hand, μ opioid receptor antagonists are drugs that block the activity of the μ opioid receptors. These receptors are also located in the brain and spinal cord and are involved in pain modulation, reward pathways, and respiratory depression. By blocking the μ opioid receptors, antagonists can counteract the effects of opioids and prevent their binding, thereby reducing pain relief and potentially reversing opioid overdose.
The combination of κ opioid receptor agonists and μ opioid receptor antagonists can be used in various therapeutic approaches. For example, in the treatment of pain, combining these two types of drugs may provide a more balanced analgesic effect with reduced risk of opioid dependence and respiratory depression. This approach aims to maximize pain relief while minimizing the potential for abuse and side effects associated with traditional opioid medications.
Overall, κ opioid receptor agonists and μ opioid receptor antagonists represent different pharmacological strategies for modulating pain and related neurological functions. Their distinct mechanisms of action make them valuable tools in the development of novel analgesic therapies and the management of opioid-related disorders.
Drug Target R&D Trends for Nalbuphine Hydrochloride
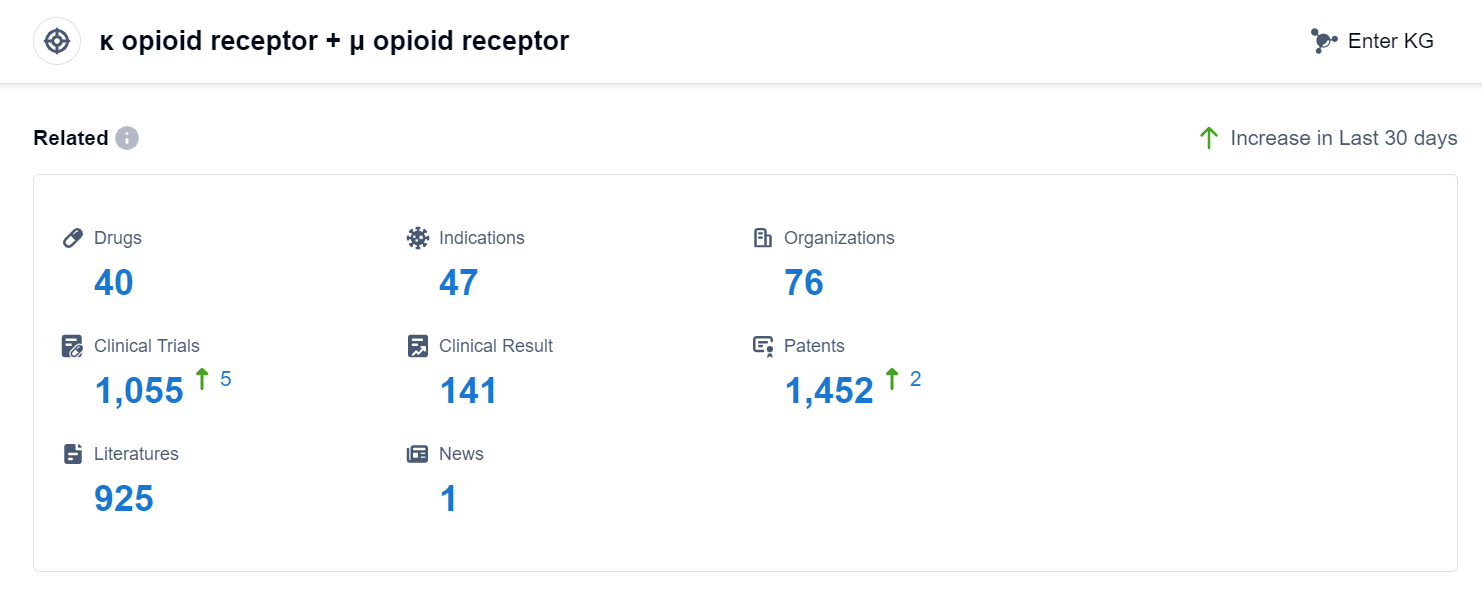
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 40 κ opioid receptor + μ opioid receptor drugs worldwide, from 76 organizations, covering 47 indications, and conducting 1055 clinical trials.
The current competitive landscape of the target κ opioid receptor + μ opioid receptor is characterized by the presence of multiple companies with varying levels of R&D progress. Indivior PLC, Lumosa Therapeutics Co., Ltd., and China are leading in terms of the number of drugs in the Approved stage. The indication analysis shows that drugs under this target have been approved for various indications, with Pain being the most common indication. The drug types progressing most rapidly are Small molecule drugs and Unknown drugs. China is a country that is developing rapidly under this target, with a significant number of drugs in the Approved stage. Overall, the target κ opioid receptor + μ opioid receptor shows promise in the pharmaceutical industry, with ongoing research and development efforts leading to the approval of drugs for various indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Nalbuphine Hydrochloride is a small molecule drug that targets the κ opioid receptor and μ opioid receptor. It is approved for use in various therapeutic areas, including nervous system diseases, other diseases, skin and musculoskeletal diseases, and respiratory diseases. The drug has multiple active indications, primarily focusing on pain management and treatment of related conditions. Its originator organization is Endo International Plc, and it has received approvals in both the United States and China. The Fast Track regulation underscores the drug's potential to address unmet medical needs and expedite its development and review process.