Deep Scientific Insights on Tofacitinib Citrate's R&D Progress, Mechanism of Action, and Drug Target
Tofacitinib Citrate's R&D Progress
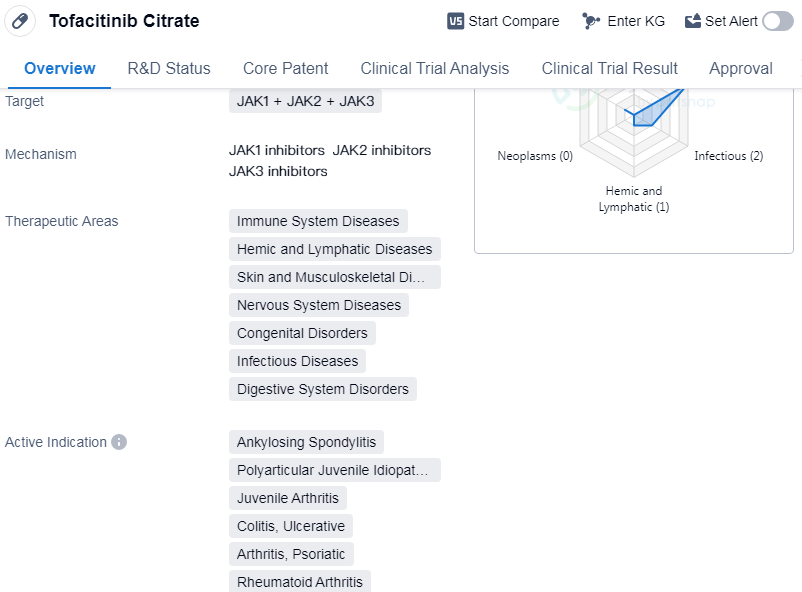
Tofacitinib Citrate is a small molecule drug that targets JAK1, JAK2, and JAK3. It has been approved for use in immune system diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, nervous system diseases, congenital disorders, infectious diseases, and digestive system disorders.The drug has shown efficacy in treating several active indications, including ankylosing spondylitis, juvenile arthritis, colitis, ulcerative, arthritis, etc.
Tofacitinib Citrate was developed by Pfizer Inc., a leading pharmaceutical company. It received its first approval in the United States in November 2012. The drug has also been approved in China, indicating its global reach and potential market.
In terms of regulatory status, Tofacitinib Citrate has undergone priority review and special review projects, suggesting its significance and potential impact in the pharmaceutical industry. These regulatory designations highlight the drug's potential to address unmet medical needs and provide innovative treatment options for patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tofacitinib Citrate: JAK1 inhibitors, JAK2 inhibitors, and JAK3 inhibitors
JAK1 inhibitors, JAK2 inhibitors, and JAK3 inhibitors are a group of drugs that target different isoforms of the Janus kinase (JAK) enzymes. These enzymes play a crucial role in the signaling pathways involved in immune responses and inflammation.
From a biomedical perspective, JAK inhibitors are pharmaceutical agents that specifically inhibit the activity of JAK enzymes. JAK1, JAK2, and JAK3 are isoforms of the JAK enzyme family, and each isoform has different functions and is expressed in different cell types.
JAK inhibitors are primarily used in the treatment of various autoimmune diseases, such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. By blocking the activity of JAK enzymes, these inhibitors help to regulate the immune response and reduce inflammation.
JAK1 inhibitors specifically target the JAK1 isoform, which is involved in cytokine signaling and immune cell activation. By inhibiting JAK1, these drugs can modulate the immune response and alleviate symptoms associated with autoimmune diseases.
JAK2 inhibitors selectively inhibit the JAK2 isoform, which is primarily involved in the signaling pathways of hematopoietic cells, including red blood cell production. These inhibitors have shown efficacy in the treatment of myeloproliferative neoplasms, a group of blood disorders characterized by abnormal cell growth.
JAK3 inhibitors specifically target the JAK3 isoform, which is predominantly expressed in immune cells, particularly T cells and natural killer cells. By inhibiting JAK3, these drugs can interfere with the signaling pathways involved in immune cell activation and reduce inflammation.
Overall, JAK1, JAK2, and JAK3 inhibitors are valuable therapeutic options for managing autoimmune diseases and certain blood disorders by selectively targeting specific isoforms of the JAK enzyme family.
Drug Target R&D Trends for Tofacitinib Citrate
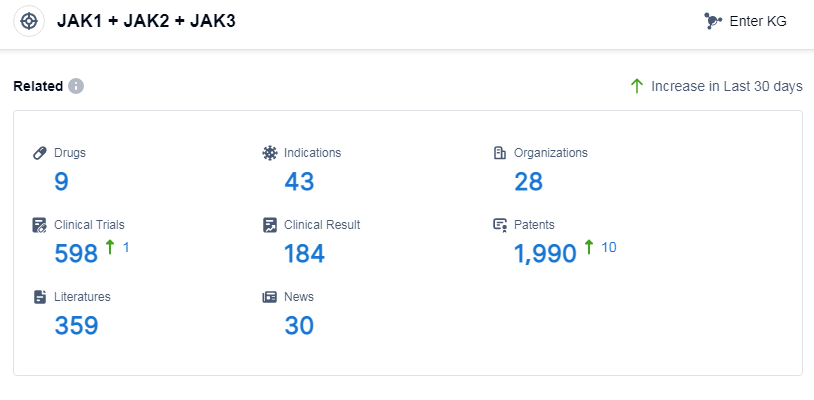
According to Patsnap Synapse, as of 8 Sep 2023, there are a total of 9 JAK1, JAK2, and JAK3 drugs worldwide, from 28 organizations, covering 43 indications, and conducting 598 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target JAK1, JAK2, and JAK3 in the pharmaceutical industry is characterized by the involvement of multiple companies, with AbbVie, Inc., Pfizer Inc., and Astellas Pharma, Inc. leading in R&D progress. The target has been approved for various indications, with rheumatoid arthritis having the highest number of approved drugs. Small molecule drugs are progressing rapidly under this target. Japan, China, United States, and European Union are the countries/locations developing fastest, with China showing significant progress. Overall, the target JAK1, JAK2, and JAK3 has a competitive landscape and promising future development potential in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Tofacitinib Citrate is a small molecule drug developed by Pfizer Inc. that targets JAK1, JAK2, and JAK3. It has been approved for various therapeutic areas and active indications. The drug received its first approval in the United States in 2012 and has also been approved in China. Its regulatory status includes priority review and special review projects, indicating its potential impact in the pharmaceutical industry.