Denali Initiates Phase 2a Trial for LRRK2 Inhibitor in Parkinson's
Denali Therapeutics Inc. (Nasdaq: DNLI) has declared the commencement of dosing in a worldwide Phase 2a clinical trial named BEACON, involving the investigational LRRK2 inhibitor BIIB122 (DNL151) for individuals diagnosed with LRRK2-associated Parkinson’s disease (LRRK2-PD). Inhibiting LRRK2 presents a promising therapeutic strategy that might mitigate the advancement of Parkinson’s disease by addressing the fundamental lysosomal dysfunction associated with the condition.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase 2a trial aims to assess the safety and biomarker effects of daily oral administration of BIIB122 in around 50 individuals diagnosed with Parkinson’s disease who have LRRK2 pathogenic mutations verified through genetic tests. The trial includes a double-blind treatment phase lasting three months, followed by an open-label extension. Denali is responsible for the Investigational New Drug application as well as overseeing the design and implementation of this Phase 2a trial. Funding for this study comes from a Collaboration and Development Funding Agreement between Denali and an external partner. Additionally, BIIB122 is being tested in the ongoing global Phase 2b LUMA trial, which focuses on early-stage Parkinson’s patients, regardless of LRRK2 mutation status, in partnership with Biogen.
“We are excited to launch this study and expand our initiatives to evaluate BIIB122 as a possible treatment for those affected by Parkinson’s disease linked to LRRK2 mutations,” stated Carole Ho, M.D., Chief Medical Officer at Denali. “We anticipate ongoing collaboration with the Parkinson’s community to generate biomarker and safety information that could clarify the potential impact of LRRK2 inhibition on the progression of this condition.”
“LRRK2 remains a critical focus within Parkinson’s research and a key area for developing disease-modifying therapies,” mentioned Todd Sherer, Ph.D., Chief Mission Officer of The Michael J. Fox Foundation. “The Phase 2a study involving BIIB122 is a significant step toward realizing the therapeutic potential of LRRK2 for individuals with Parkinson’s disease.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 10, 2024, there are 74 investigational drugs for the LRRK2 target, including 21 indications, 60 R&D institutions involved, with related clinical trials reaching 25, and as many as 4251 patents.
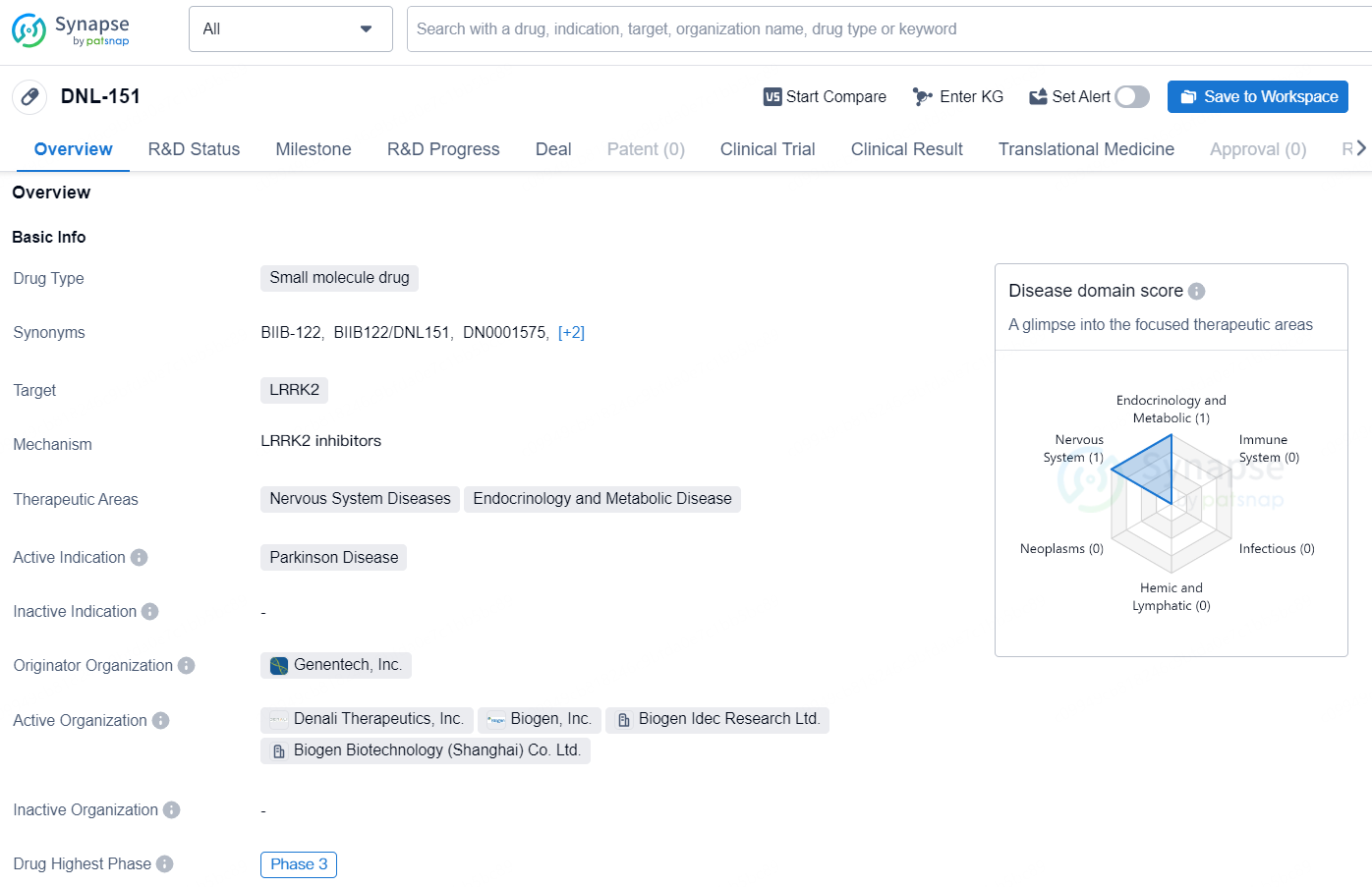
DNL-151 is a small molecule drug developed by Genentech, Inc. that targets LRRK2, and it is currently in the highest Phase of development for Parkinson's Disease treatment globally, at Phase 3. In China, the drug has progressed to Phase 2.