Disc Medicine Launches Phase 1 Trial of DISC-3405 with Healthy Participants
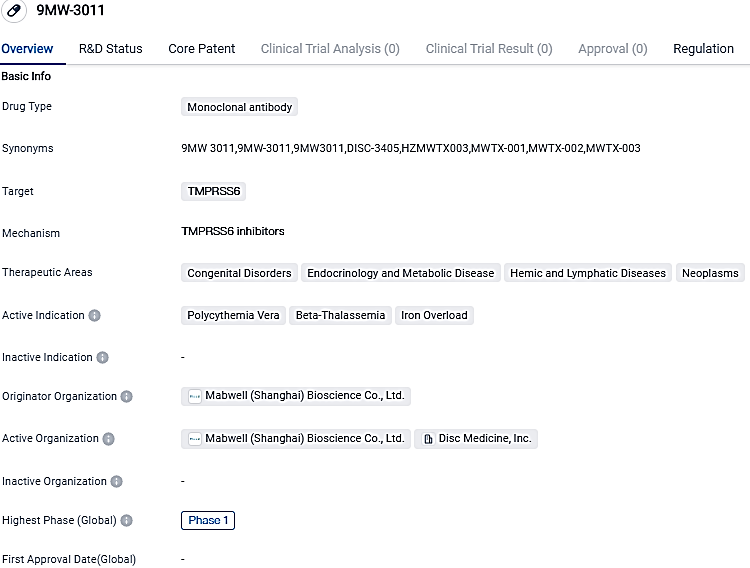
Disc Medicine, Inc., a biotech business in the clinical trial stage that's concentrating on identifying, creating, and marketing unique remedies for severe blood-related illnesses, has revealed the start of a Phase 1 trial for DISC-3405 (formerly MWTX-003) with healthy participants.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
DISC-3405 is a specially made monoclonal antibody that aims to target TMPRSS6 (Transmembrane Serine Protease 6, or Matriptase-2) with the goal of boosting hepcidin and lowering iron levels.
“With the start of this trial, Disc successfully introduces its third clinical program, solidifying our standing in the area of hepcidin biology and iron equilibrium. We consider DISC-3405 to have wide-ranging applicability in various conditions where iron regulation could provide a medical advantage, such as polycythemia vera, iron surplus syndromes and numerous other disorders.” commented John Quisel, J.D., Ph.D., the President and CEO of Disc.
“Upon this trial's conclusion, we are eager to explore these applications. This significant step arrives at a thrilling period for our organization as we anticipate revealing data insights from the ongoing clinical assessments of our other two initiatives, bitopertin and DISC-0974, within the year.” Further, John Quisel expounded.
The scrutiny will pursue a randomized, double-masked, placebo-managed, single- and multiple-rising dose Phase 1 trial in healthy volunteers, aiming to assess safety, tolerability, pharmacokinetics, and indications of pharmacodynamic action, including indicators of iron equilibrium and erythropoiesis.
The single-rising dose fragment of the analysis is projected to register four batches at escalating dosage quantities, commencing with intravenous administration with an approach to shift to subcutaneous administration later in the study. The multiple-rising dose fragment is foreseen to register two batches, each undergoing subcutaneous injections of DISC-3405. Upon the completion of this scrutiny, Disc projects to launch a trial in polycythemia vera, a condition for which DISC-3405 has been granted Fast Track Designation.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
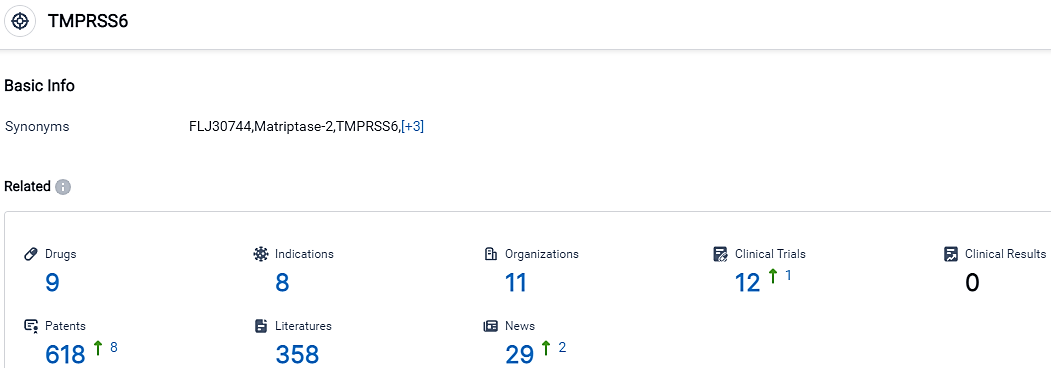
According to the data provided by the Synapse Database, As of October 14, 2023, there are 9 investigational drugs for the TMPRSS6 target, including 8 indications, 11 R&D institutions involved, with related clinical trials reaching 12,and as many as 618 patents.
DISC-3405, formerly known as MWTX-003, is an investigational, anti-TMPRSS6 monoclonal antibody designed to increase hepcidin production and suppress serum iron, that Disc in-licensed from Mabwell Therapeutics in January 2023. Its Fast Track designation and progress in clinical development indicate that it is an area of interest in the pharmaceutical industry, with potential for addressing unmet medical needs in these therapeutic areas.