Discussing the invalidation of parts of Upadacitinib patents and the dissolution of Reistone Biopharma, a subsidiary of Jiangsu Hengrui Pharmaceutical
On the night of September 14, market rumors indicated that Jiangsu Hengrui Pharmaceutical was about to dissolve its subsidiary, Reistone Biopharma, completely - reportedly having seized the company seal, frozen bank accounts, and offered employees an N+1 compensation package. A Hengrui insider responded saying that the company is awaiting official information on the matter. However, given Reistone Biopharma's relatively small scale with only a hundred or so employees, the impact on Hengrui is expected to be limited.
Additionally, there were reports that two months ago, Reistone Biopharma planned to return its product pipeline to Hengrui Pharmaceutical, with Hengrui withdrawing its stock in Reistone Biopharma. But this news has not been officially confirmed by Hengrui.
Regarding the reasons for Reistone's dissolution, speculation abounds. A plausible explanation is that a China-specific compound patent for Upadacitinib has been invalidated, thus potentially paving the way for earlier market entry of generic versions of the drug. Reistone's core asset in its clinical pipeline is its JAK inhibitor, SHR0302 (Fosravuconazole). Reistone had submitted a marketing authorization application for the treatment of moderate to severe atopic dermatitis to the drug regulatory authority in June and this was accepted. However, as announced by Hengrui on August 25, the application covering this indication has been voluntarily withdrawn.
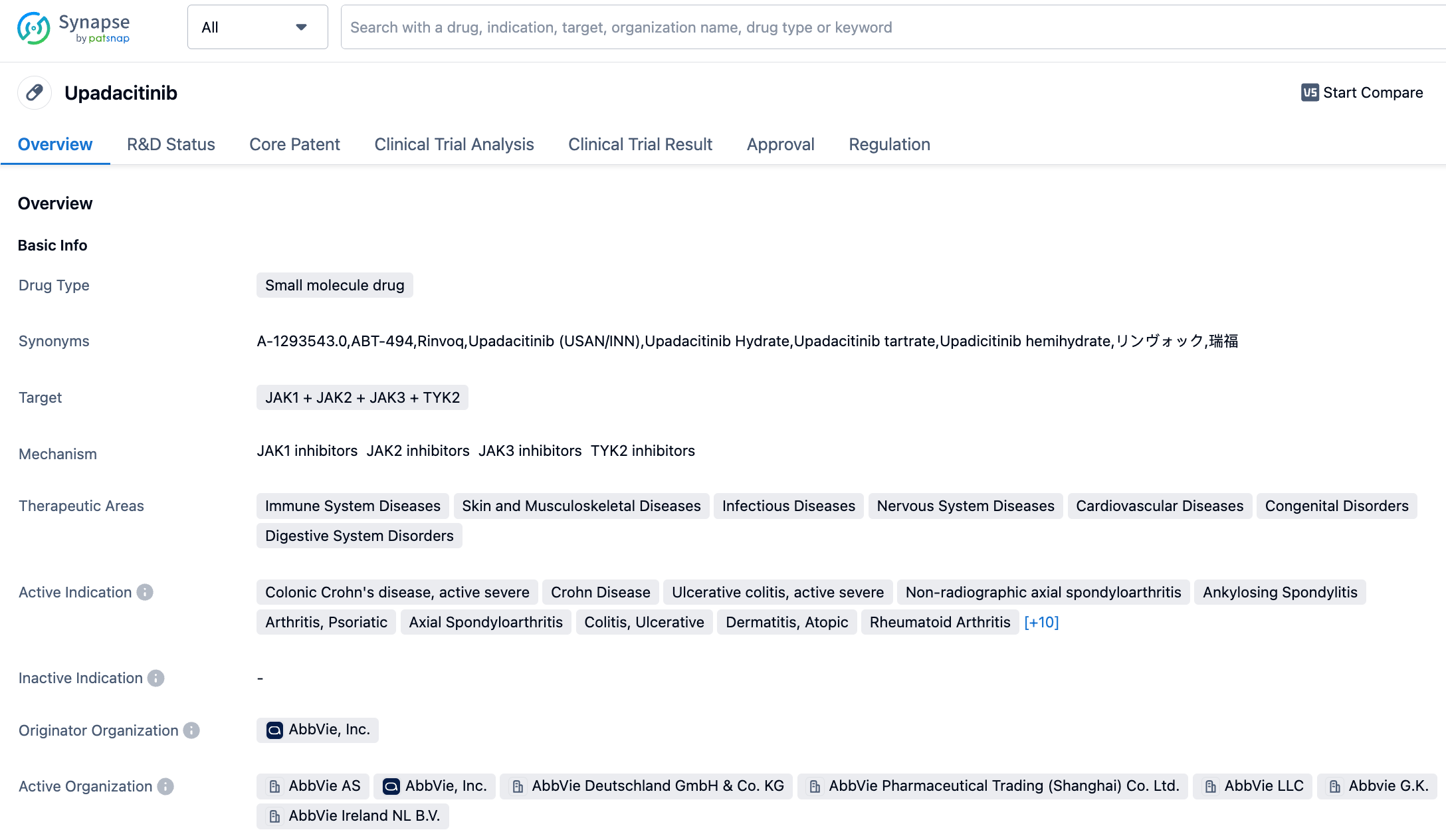
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of upadacitinib.
By mid-2023, the cost Hengrui incurred in developing SHR0302 reached 760 million RMB. Additionally, it had multiple indications undergoing phase III clinical trials, including Psoriatic Arthritis, Ankylosing Spondylitis, Rheumatoid Arthritis, Ulcerative Colitis, moderate to severe Atopic Dermatitis, and Alopecia Areata.
Likewise, a JAK1 inhibitor, Upadacitinib, apart from a phase III Up-AA trial (PO, tablets) for Alopecia Areata to be conducted in adults and adolescents in October 2023, was already in the market for all other indications for which SHR0302 was undergoing phase III trials.
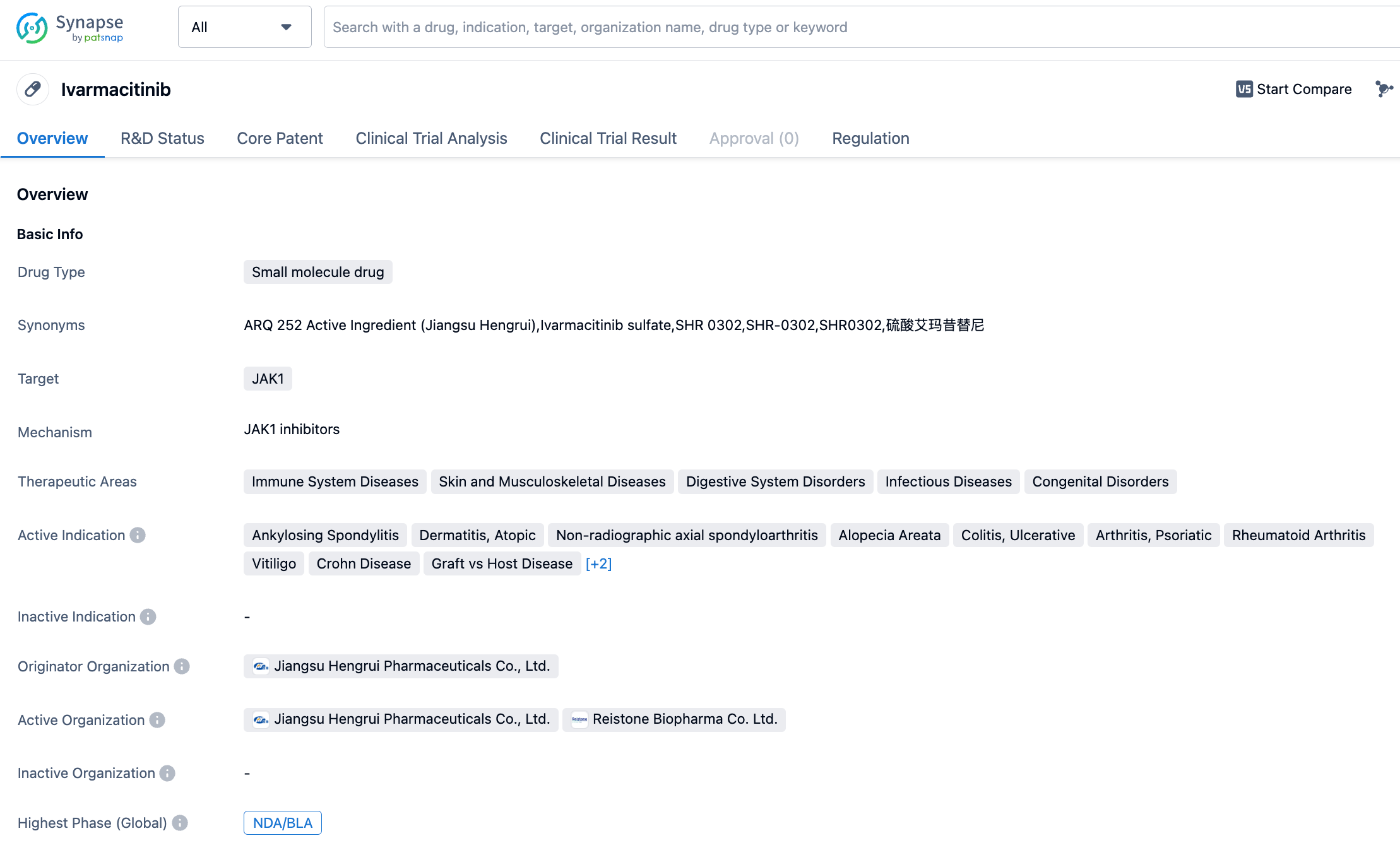
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of ivarmacitinib.
Since 2019, when Upadacitinib entered priority review and was approved for Rheumatoid Arthritis, the range of new indications has continued to grow due to the robust therapeutic effects of the JAK-STAT signaling pathway.
The market has also highly recognized Upadacitinib. In 2021, the global sales of Upadacitinib reached $1.651 billion; in the first half of 2023 alone, global sales had already reached $1.604 billion. Up to now, no domestic pharmaceutical company has registered to market a generic version of Upadacitinib.
With the invalidation of some patents for Upadacitinib compounds, the competition Hengrui's SHR0302 faces is not only from Upadacitinib but also from a large number of Upadacitinib generics. Can SHR0302 compete with generic Upadacitinib? The answer is probably already clear to everyone.
The chemical compound patent of Upadacitinib has been invalidated
The National Intellectual Property Administration of China issued a document on August 10, 2023, according to the provisions of Article 46, Paragraph 1 of the Patent Law. The National Intellectual Property Administration reviewed the invalidation request submitted by the invalidation petitioner (Sichuan Guowei Pharmaceutical Company) for the above-mentioned patent right (ZL201080062920.6) and made the following decision: The patent right is announced entirely invalid.
Based on the text submitted by the patent holder (AbbVie) on February 21, 2023, patent number 201080062920.6 is declared to be entirely invalid.
Part of AbbVie's Chinese patent 201810902092.0 has been invalidated. At the moment, only the chemical compound patent has been found to be wholly invalid, and part of the composition patent is invalid. However, the patents related to crystal forms and salt forms can still prevent the marketing of generic drugs. Still, with the most robust position of moats broken, in the future, more companies might be able to bypass the process patent and successfully market Upadacitinib generics.
Should any party object to this decision, they may, in accordance with the provisions of Article 46, Paragraph 2 of the Patent Law, file a lawsuit with the Beijing Intellectual Property Court within three months from the date of receipt of this decision. As per the stipulation, once one party files a case, the other party may participate in the proceeding as a third party.
Based on the data of overturned cases in previous years, the likelihood of successfully overturning the case is relatively low, below 20%.
Let's take a look at the invalidated patent for AbbVie's compound, patent number ZL201080062920.6, also known as WO 2011/068881A1 titled "Novel Tricyclic Compounds". The patent sought protection for the compound structure of Upadacitinib and its therapeutic use in immuno-oncological diseases. It was first published on October 3, 2012; substantive examination started on January 9, 2013; there was a transfer of application rights on July 10, 2013, from Abbott Laboratories to AbbVie, after which the patent was granted on December 3, 2014.
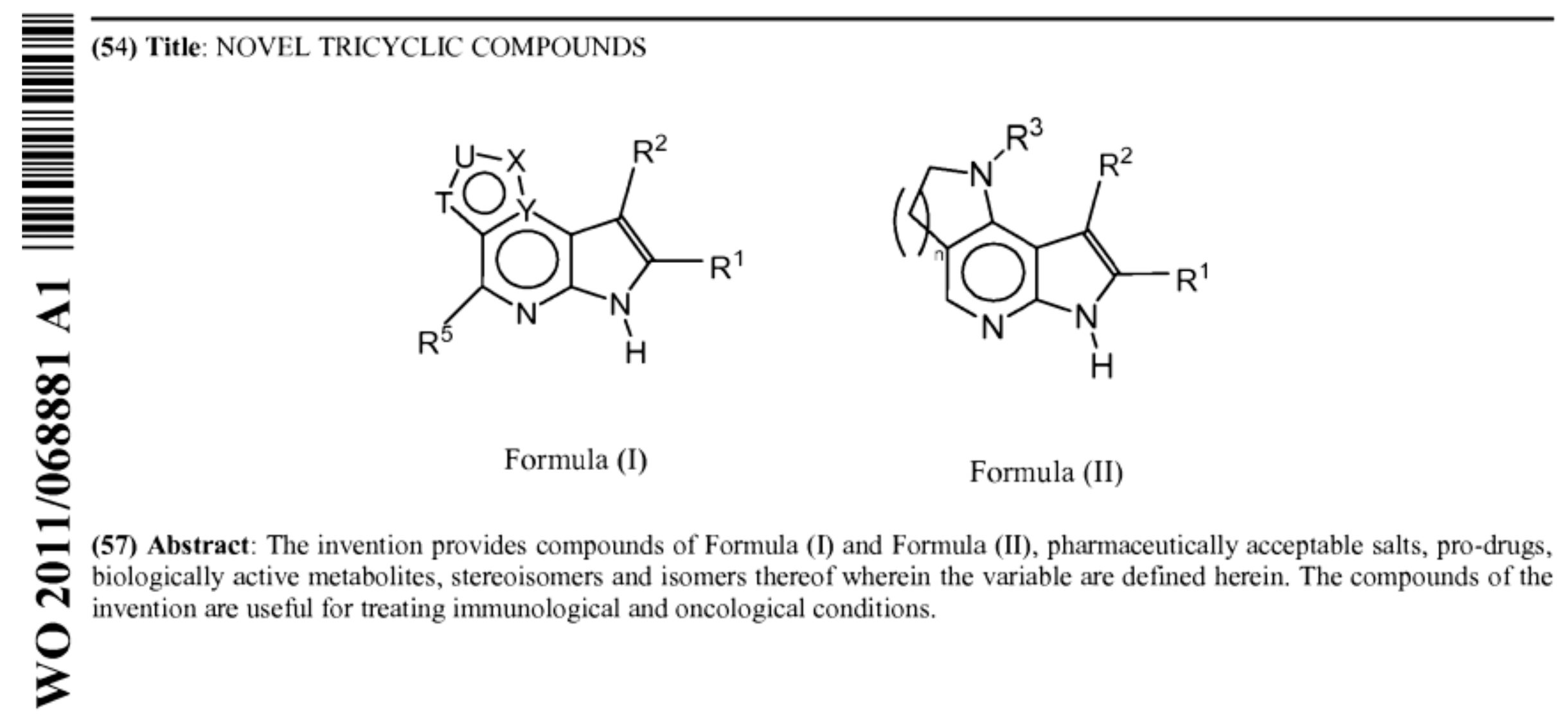
This patent document can be downloaded from the Synapse database. With several embodiments included in the patent, the core compound Upadacitinib was found exemplified on page 363. However, there were limited published data for Upadacitinib - just including synthesis, molecular weight, retention time from chiral separation column, lacking in detailed efficacy data. A review of the structures explored in the patent embodiments suggests a focus on sulfonamide compounds rather than a urea structure like Upadacitinib. This may indicate that AbbVie's primary compound of interest at that time wasn't Upadacitinib. The insufficient disclosure of the instruction manual containing the core compound resulted from this.
Furthermore, there is significant overlap in the definitions of the generic formula Ic of the invalidated patent and the generic formula Ic of reference 2 (WO 2009/152133A). Instance 19 and instance 16 of the specific compounds disclosed in reference 2 appear as examples in this patent specification, and Upadacitinib, protected by the implicated patent, falls within the protection scope of both. This is contrary to the priority principle of compound patents. Regardless of whether one judges from a creativity standpoint or the insufficient disclosure in the specification, the pharmaceutical use patent for Upadacitinib lacks inventiveness.
It's worth mentioning that the applicant of reference 2 is Abbott Pharmaceuticals, the right holder before the change of the implicated patent. This is the closest prior art to Upadacitinib. It might also indicate that at the time of research and development, Upadacitinib was not the compound they intended to push forward, as there is not meticulous coverage for protection.
However, the application of a patent and the subsequent addition of data cannot be conducted after a certain timeframe, making it difficult to rectify any omissions that occur subsequently.
Summary
Drug development is characterized by its lengthy process and substantial investment. Therefore, patent applicants often choose to submit a series of applications to protect their phased research results. Subsequent applications are typically based on prior applications and involve selective inventions or improvements, inevitably leading to some degree of overlap or redundancy in protective scope.
The system of priority rights, the patent holder's strategy of division applications, and the peculiar drafting style of Markush claims in the field of chemistry further exacerbate the difficulty of determining the patentability of the subject matter of protection.
At this point, it is crucial not only for the applicant to adequately disclose the purpose of the invention, the technological means adopted, and the technological results obtained in the application documents but also for them to clearly express the technical effects achieved through improvements or selective inventions. This will greatly benefit subsequent inventions in obtaining appropriate patent protection.
From the perspective of patent granting, in such series applications for drug compounds, if general purposes of invention and compound descriptions in the specifications are permitted while lacking necessary effect data, and if evidence of specific technical effects of certain compounds mentioned vaguely in the specifications can be proved by providing supplemental experimental data, this effectively encourages the applicant to "encircle land" by repeatedly submitting overlapping Markush general formulas within a broad range. This indirectly extends the protection period and potentially allows subsequent research results to be reflected in the prior invention in the form of supplementary data. This, undeniably, results in unfairness for other researchers in the field and the general public.