Drug pipeline database guide for pipeline & marketed drug analysis
Introduction
The proliferation and advancement of the pharmaceutical and biotech sectors have led to the necessity for comprehensive and intricate tools to provide invaluable insights into drug discovery and development. Consequently, the proficiency and versatility of the drug pipeline database have emerged as an indispensable resource for this significant task. As a guide to this noteworthy tool, this article will delve into the myriad features and applications of a drug pipeline database, particularly discussing Synapse, one such database that sets the bar high. Synapse can provide us with in-depth and analytical intelligence in various aspects of drug discovery and development in the pharmaceutical and biotechnology industries.
Data Coverage and source in Synapse drug pipeline database
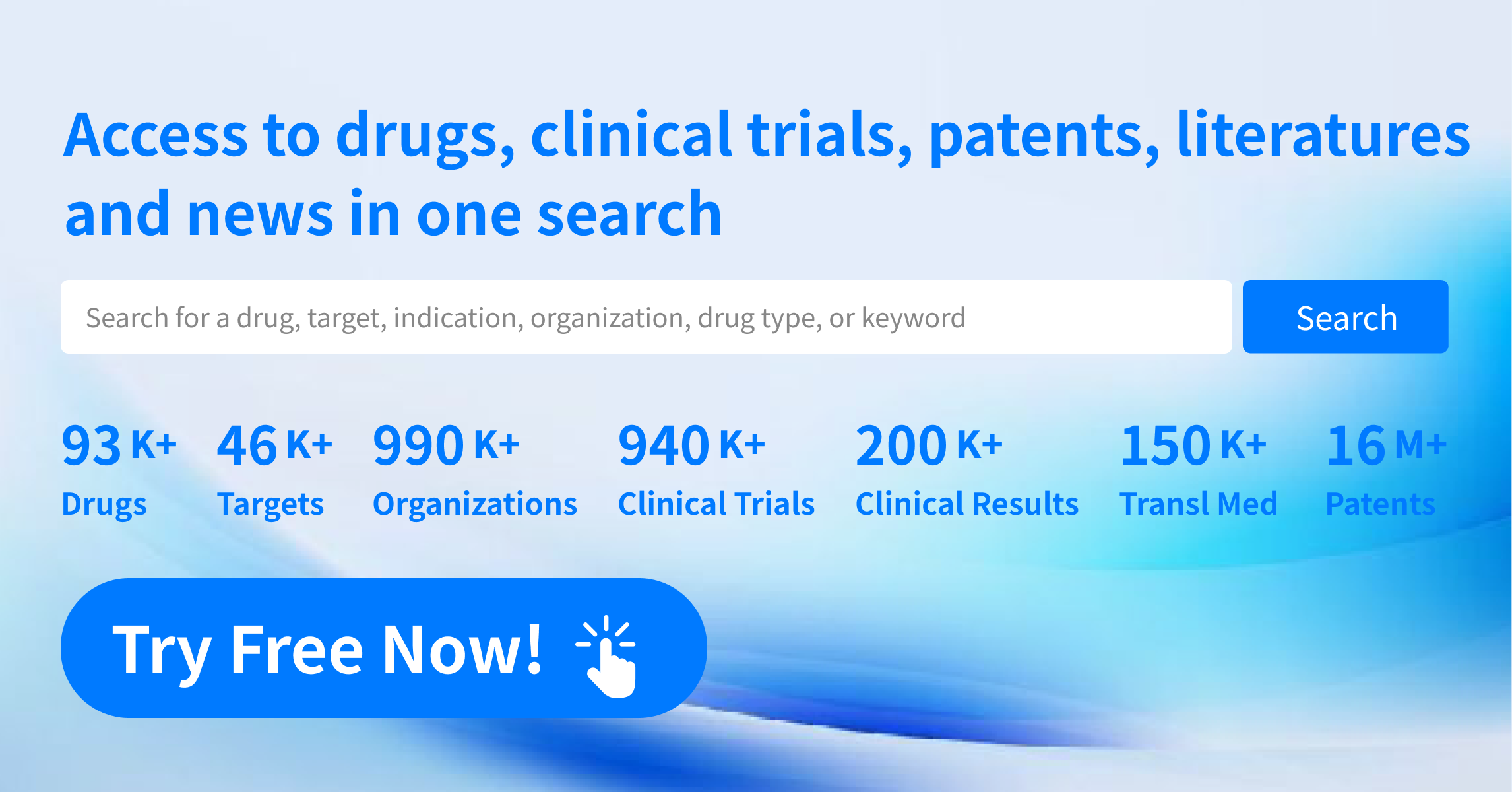
Leveraging on Synapse's pioneering "Global Big Data AI Real-Time Mining + Pharmaceutical Expert Team Verification" intelligence processing mechanism, Synapse have established a global real-time data update system in drug pipeline database, covering over 45,000+ targets, 73,000+ new drugs, 362,000+ organizations, 800,000+ clinical trials, 60,000,000+ selected literature, and 13,000,000+ drug patents.
The drug information database provides real-time monitoring of the global biopharmaceutical industry trends, updated daily to ensure dynamic drug information updates, with primary data sources including:
● Global mainstream drug evaluation agencies such as FDA/EMA/PMDA/NMPA/CDE etc.
● Major regional clinical institutions such as United States Clincal trials/WHO (ICTRP)/European Union (EU-CTR)/Japan (NIPH/UMIN-CTR/JMACCT CTR)/China (CTR/chiCTR) etc.
● Biopharmaceutical news and information, as well as development pipelines of global biopharmaceutical company official websites
● Global patents
● Life sciences related literature and other sources.
Application scenarios of Synapse drug pipeline database
You can use Synapse drug pipeline database con planning new drug pipelines, setting company strategic direction, looking for new drug research and development opportunities, evaluating the value of project introductions, selecting investment targets, tracking competitor strategies, and developing new customers.
Free access with example guidance on utilizing the Synapse drug pipeline database
By simply entering a company's keyword in the search box on the Synapse webpage, you can swiftly acquire a comprehensive overview of the company's pipeline layout, intricate details about its specialization in the disease field, in-depth insights into the technology utilized most frequently in drug applications, along with the names of the top five targets commonly developed by the company and the quantity of associated drugs, all without the necessity to log in or register.
Here's a demo example of how to use 'Pfizer' as a keyword in a search:
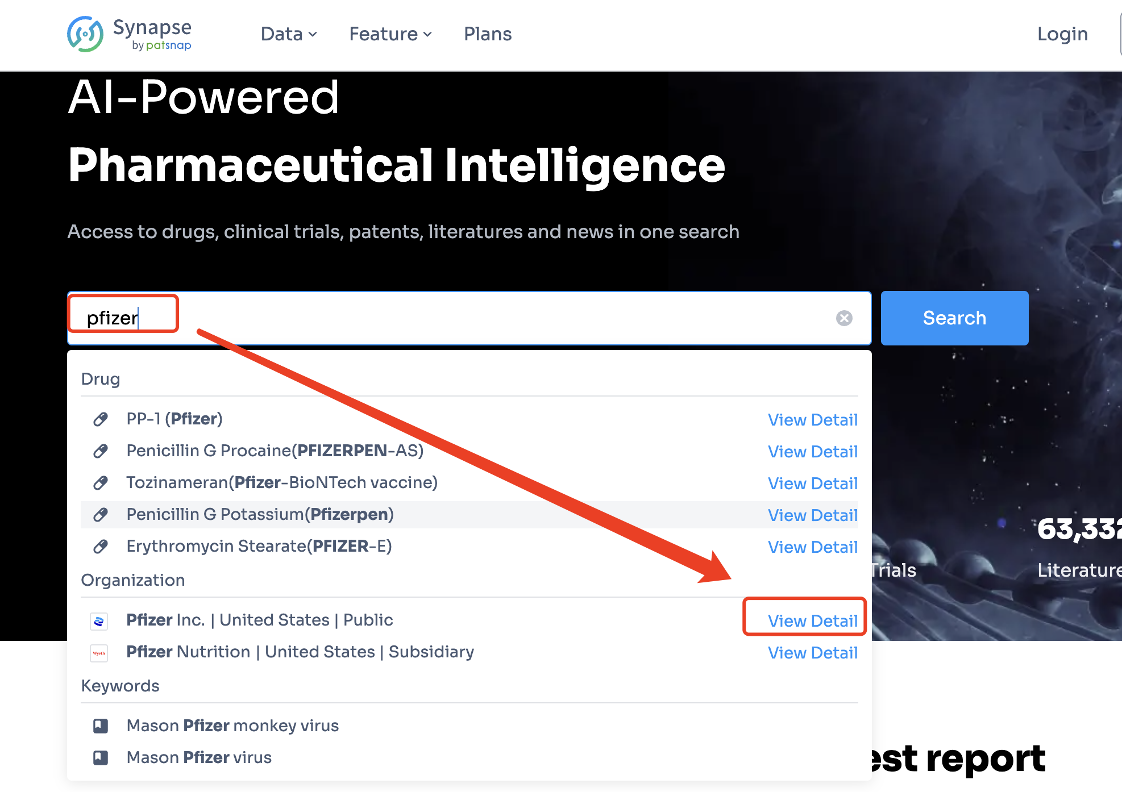
Step 1: Open the Synapse webpage and enter 'Pfizer' into the search box as illustrated in the picture. Then, click on 'View Detail'. Alternatively, you can directly open the permanent link to the Pfizer pipeline.
https://synapse.patsnap.com/organization/9597e75ba30c9958d4331f586407d7cf
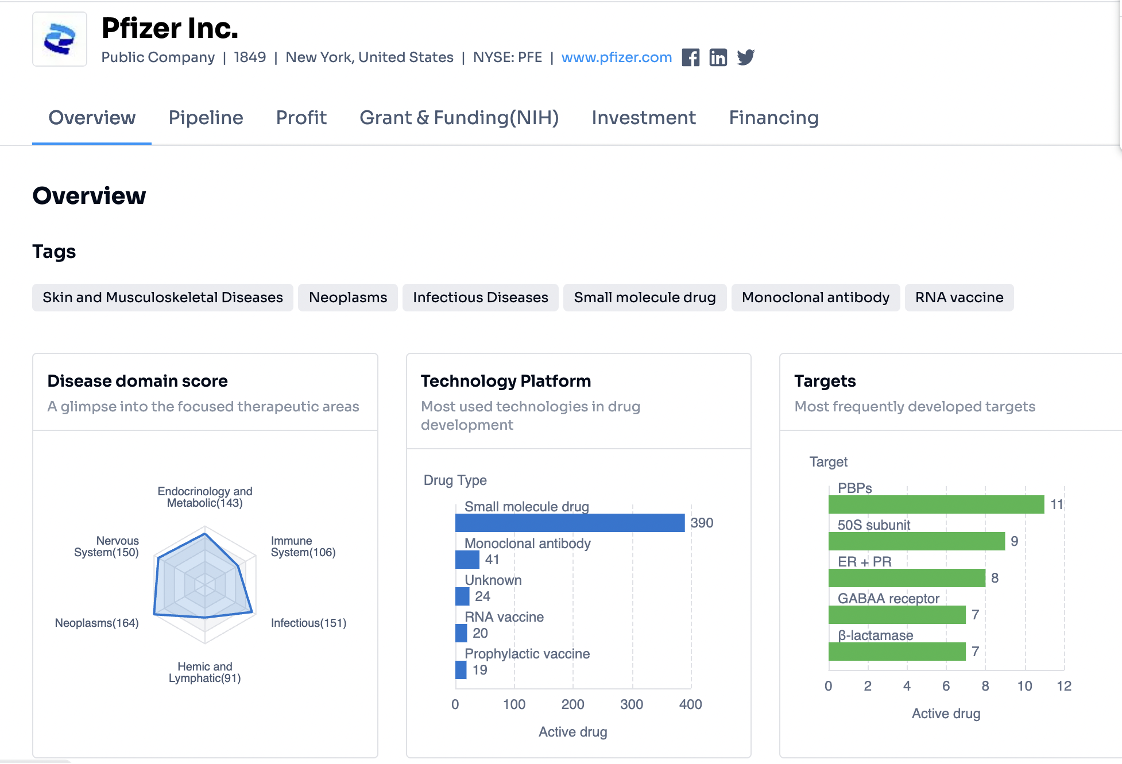
Step 2: In the link you have opened, Pfizer's summary information is available to browse the key highlights of current drug development from the collected resources.
● The recorded count for disease domains represents the number of ongoing drug developments focusing on a specific category of diseases. For example, Neoplasms or tumours presently top the list with 164 active development instances, closely trailed by infectious diseases (151), and diseases related to the nervous system (150). This data suggests that these sectors are currently the central point of attention in drug development.
● When it comes to drug types, the lion's share of developments involves small molecule drugs (390), while monoclonal antibodies stand as a less common option (41).
● PBPs (bacterial penicillin-binding proteins) and the 50S subunit (50S ribosomal subunit) of the bacterial ribosome are the most popular drug targets, primarily used for formulating antibiotic drugs. This trend highlights the pressing requirement to devise new interventions for bacterial infections.
● The technology platforms deployed for these developments remain unspecified. However, the citation of RNA vaccines implies that RNA technology, along with potentially other biotechnological approaches, are being implemented.
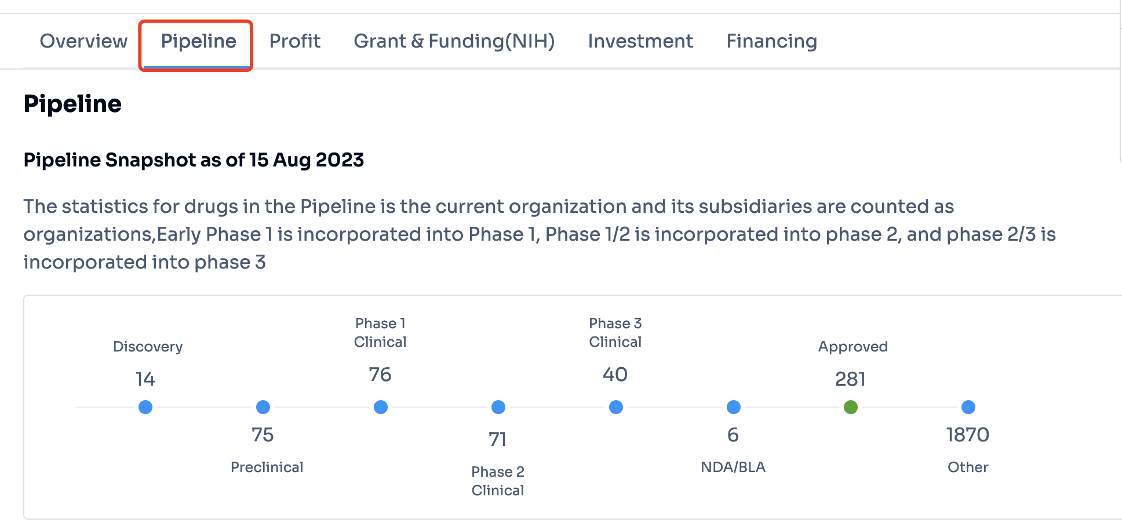
Step 3:
Select the word "pipeline" highlighted in the image below to swiftly access comprehensive information regarding Pfizer's latest pipeline drugs and marketed drugs at different developmental stages. To see more detailed information like the names of the drugs, their targets, clinical trial data, patent, indication and news, click on the numerical figure shown in the image.
Conclusion
The Synapse Drug Pipeline Database offers a comprehensive compilation of drug data throughout the R&D lifecycle. This includes everything, from discovery and clinical development to regulatory submission and commercialization. It offers profound industry insight and the database is seamlessly integrated and continually updated to provide a comprehensive perspective of the drug landscape for each company or indication. Every pharmaceutical developer, decision maker, analyst, and Business Developer should utilize the Synapse drug pipeline database as a tool.