Cyfendus - A dual-dose anthrax vaccine for post-exposure prevention of anthrax
Anthrax Vaccine Adsorbed, Adjuvanted, com-mercially known as CYFENDUS, is a preventive vaccine targeting TLR9. This vaccine was developped by Emergent BioSolutions, Inc. and was approved by FDA in the United States on July 20, 2023.
It is used for post-exposure prophylaxis of disease following suspected or confirmed exposure to Bacillus anthracis in persons aged 18 through 65 when administered in conjunction with recommended antibacterial drugs. The efficacy of CYFENDUS for postexposure pro-phylaxis is based solely on studies in animal models of inhalational anthrax.
This drug was granted Fast Track designation by the FDA as early as 2011, later received Orphan Drug designation in 2014, and obtained Emergency Use Authorization from the FDA in 2019. Four years later, Emergent has won a full approval from the FDA for its use in adults 18 to 65 in conjunction with antibacterial drugs, after suspected or confirmed exposure to the potentially fatal bacteria.
Anthrax is a serious infectious disease caused by spores of the bacterium, Bacillus anthracis (B. anthracis). The B. anthracis spores are resistant to destruction and are easily spread in the air. In some countries, anthrax is naturally present in the soil, which can infect grazing animals that ingest the spores. The most common form of anthrax infection in humans is through the skin (cutaneous). This can happen when a person has a cut and handles infected animals or contaminated animal products like wool, hides, or hair. The death rates from anthrax infection without treatment vary, depending on the exposure type and are approximately 20% for cutaneous anthrax, over 50% for gastrointestinal anthrax; and inhalation anthrax is almost always fatal. All types of anthrax infection can be treated with certain antibiotics.
Mechanism of Action
TLR9 is an aspartic acid-rich endoplasmic reticulum receptor and a member of the "Toll-like receptors (TLRs)" family. TLR9 is mainly present in immune cells such as macrophages, dendritic cells, B cells, and epithelial cells and can recognize and bind to DNA from viruses, bacteria, fungi, and parasites. TLR9 identifies the dynamic methylation pattern of CpG in DNA, triggering downstream immune responses. Anthrax is an infectious disease caused by the Gram-positive, spore-forming bacterium B. anthracis. CYFENDUS induces antibodies against protective antigen protein that may contribute to protection by neutralizing the activities of the cytotoxic lethal toxin and edema toxin of B. anthracis.
Application
In the United States, human cases of anthrax are rare. Anthrax is considered one of the more likely agents to be used in a biological attack. The vaccines have been purchased by the federal government and are stored in the Strategic National Stockpile (SNS) to be used for post-exposure prophylaxis with antibiotics in the event of a terrorist attack with anthrax. Likewise, because of biological warfare threats, the military has an active vaccination program against anthrax for personnel going to specific arenas around the world.
Core Clinical Trials
A multicenter, randomized, active-controlled, double-blind, parallel-group clinical study (Study 4; NCT03877926) was conducted to evaluate the immunogenicity and safety of a post-exposure administration schedule of two doses (0.5 mL) of CYFENDUS intramuscularly two weeks apart in adults ages 18 through 65 years.
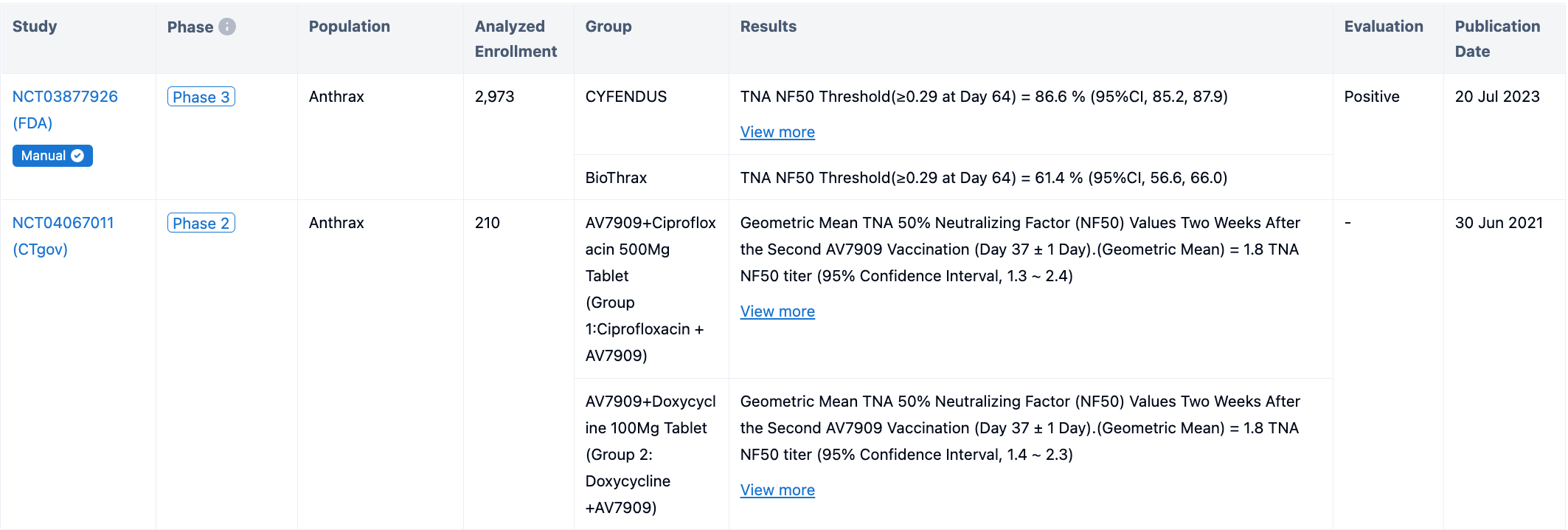 In the study, 3151 participants received at least one dose of CYFENDUS, and 533 participants received at least one dose (0.5 mL) of the comparator licensed anthrax vaccine, BioThrax. Participants were followed for immunogenicity up to Day 64. The primary immunogenicity objectives were to assess CYFENDUS vaccine-induced TNA 50% neutralization factor (NF50) response at Day 64 and non-inferiority to BioThrax following two doses of CYFENDUS administered intra-muscularly two weeks apart (Week 0 and 2) and three doses of BioThrax administered subcu-taneously two weeks apart (Week 0, 2, and 4). 66.3% of CYFENDUS participants from the PP population achieved threshold TNA NF50 value ≥0.56 on Day 64 in the study; the lower bound of the 95% CI was 64.4%. The percent of participants with a threshold TNA NF50 of ≥0.29 at Day 64 was 86.6% in the CYFENDUS group compared with 61.4% in the BioThrax group, corresponding to a difference of 25.2%. The lower bound of the 2-sided 95% CI for the difference was 20.5%, thereby demonstrating non-inferiority of CYFENDUS to BioThrax.
In the study, 3151 participants received at least one dose of CYFENDUS, and 533 participants received at least one dose (0.5 mL) of the comparator licensed anthrax vaccine, BioThrax. Participants were followed for immunogenicity up to Day 64. The primary immunogenicity objectives were to assess CYFENDUS vaccine-induced TNA 50% neutralization factor (NF50) response at Day 64 and non-inferiority to BioThrax following two doses of CYFENDUS administered intra-muscularly two weeks apart (Week 0 and 2) and three doses of BioThrax administered subcu-taneously two weeks apart (Week 0, 2, and 4). 66.3% of CYFENDUS participants from the PP population achieved threshold TNA NF50 value ≥0.56 on Day 64 in the study; the lower bound of the 95% CI was 64.4%. The percent of participants with a threshold TNA NF50 of ≥0.29 at Day 64 was 86.6% in the CYFENDUS group compared with 61.4% in the BioThrax group, corresponding to a difference of 25.2%. The lower bound of the 2-sided 95% CI for the difference was 20.5%, thereby demonstrating non-inferiority of CYFENDUS to BioThrax.
Competitive Landscape
FDA has approved two vaccines to prevent disease in persons 18 through 65 years of age following suspected or confirmed Bacillus anthracis exposure, when administered in conjunction with recommended antibacterial drugs. BioThrax is also approved for pre-exposure prophylaxis of disease in persons 18 through 65 years of age who are at high risk of exposure. Cyfendus is Emergent BioSolutions’ follow-up to its previous anthrax vaccine BioThrax. Under a five-year deal worth $1.25 billion, the company supplied 44.7 million doses to the Strategic National Stockpile between 2011 and 2016. Another five-year deal followed in which Emergent signed up to provide 29.4 million doses. In addition to the 2 vaccines, Emergent has two treatments for anthrax in its portfolio—Anthrasil, a polyclonal antibody thera-peutic, and raxibacumab, a monoclonal antibody.
According to Synapse, as of August 5, 2023, there are a total of 77 TLR9 products worldwide, from 80 institutions, covering 119 indications, and conducting 353 clinical trials. Among these products, 11 are vaccines.