Dupixent® significantly reduced COPD exacerbations in a successful Phase 3 trial, accelerating its FDA submission as a potential first approved biologic for this serious condition
The second Phase 3 research trial of Dupixent® (dupilumab) for chronic obstructive pulmonary disease has demonstrated that Dupixent notably decreased exacerbations, corroborating the positive findings previously published from the groundbreaking Phase 3 BOREAS study. The NOTUS trial also established that Dupixent treatment resulted in swift and substantial enhancements in lung function by the 12-week mark, which persisted at 52 weeks.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the NOTUS study, Dupixent's experimental application was scrutinized against a placebo in adults currently receiving maximum standard-of-care inhaled therapy who have unregulated COPD and evidence of type 2 inflammation. This interim analysis yielded remarkable efficacy for the primary endpoint, hence it will serve as the primary review of the study. Sanofi and Regeneron aim to present the replicated study's data, including noteworthy findings from the Phase 3 BOREAS study, to the FDA within the year.
For the first time ever, a biologic under investigation in COPD revealed a significant and clinically impactful reduction in two Phase 3 studies' exacerbations. This could speed up getting Dupixent to the patients needing it most since no new improvements have been discovered in more than ten years," said Naimish Patel, M.D., Sanofi's Head of Global Development, Immunology and Inflammation.
In further comments, Dr. Patel added that these findings endorse our conviction that Dupixent can revolutionize the treatment of moderate-to-severe COPD, considering the significant unmet needs for patients with uncontrolled COPD. If successful, Dupixent and itepekimab could potentially serve as treatments for about 80% of individuals suffering from moderate-to-severe COPD with recurrent exacerbations.
Earlier in the year, the FDA awarded Dupixent Breakthrough Therapy status as an add-on maintenance therapy in adult patients with unregulated COPD linked to a history of exacerbations and an eosinophilic phenotype, based on BOREAS's promising outcomes.
Dupixent, a completely human monoclonal antibody, blocks the signaling of the interleukin-4 and interleukin-13 pathways and isn't an immunosuppressant. Dupixent's development program has proven considerable clinical benefit and a reduction in type 2 inflammation in Phase 3 studies, revealing that IL-4Rα and IL-13 are critical and central contributors to type 2 inflammation that has a substantial role in multiple interconnected and frequently co-morbid diseases.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
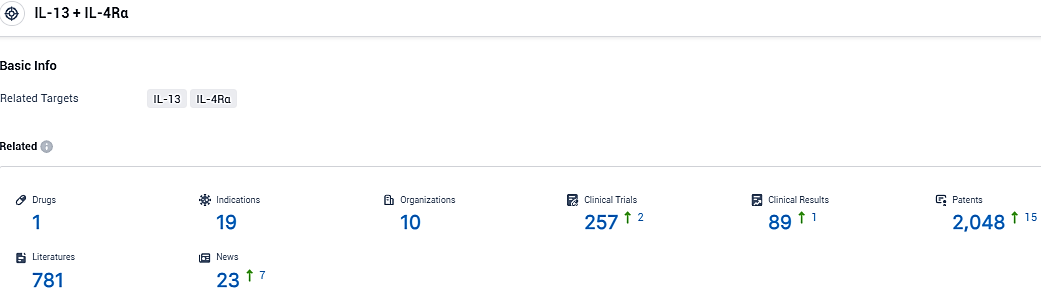
According to the data provided by the Synapse Database, As of November 30, 2023, there are 1 investigational drugs for the IL-4Rα and IL-13 target, including 19 indications, 10 R&D institutions involved, with related clinical trials reaching 257, and as many as 2048 patents.
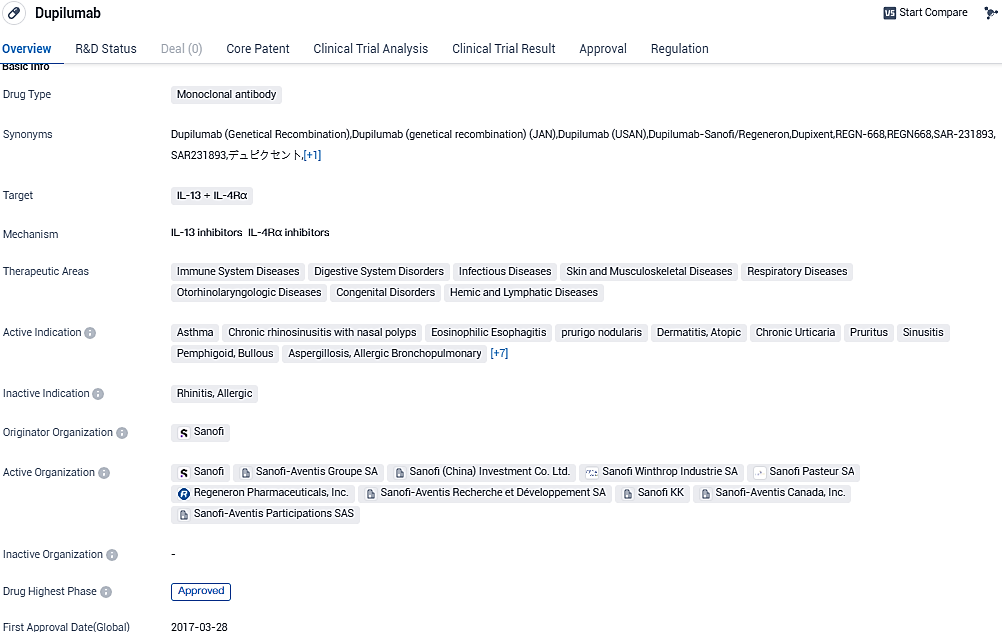
Dupixent has received regulatory approvals in one or more countries around the world for use in certain patients with atopic dermatitis, asthma, CRSwNP, EoE or prurigo nodularis in different age populations. Dupixent is currently approved for one or more of these indications in more than 60 countries, including in Europe, the U.S. and Japan. Approximately 750,000 patients are being treated with Dupixent globally.