Dupixent® Gets FDA Approval for Eosinophilic Esophagitis Treatment in Pediatric Patients
The FDA in the United States has sanctioned the use of Dupixent (dupilumab) as a therapy for young patients between the ages of 1 and 11 who have a body weight of 15 kilograms or more and are diagnosed with eosinophilic esophagitis. With this authorization, Dupixent becomes the singular treatment available in the U.S. market that directly targets this condition for the mentioned pediatric age group.
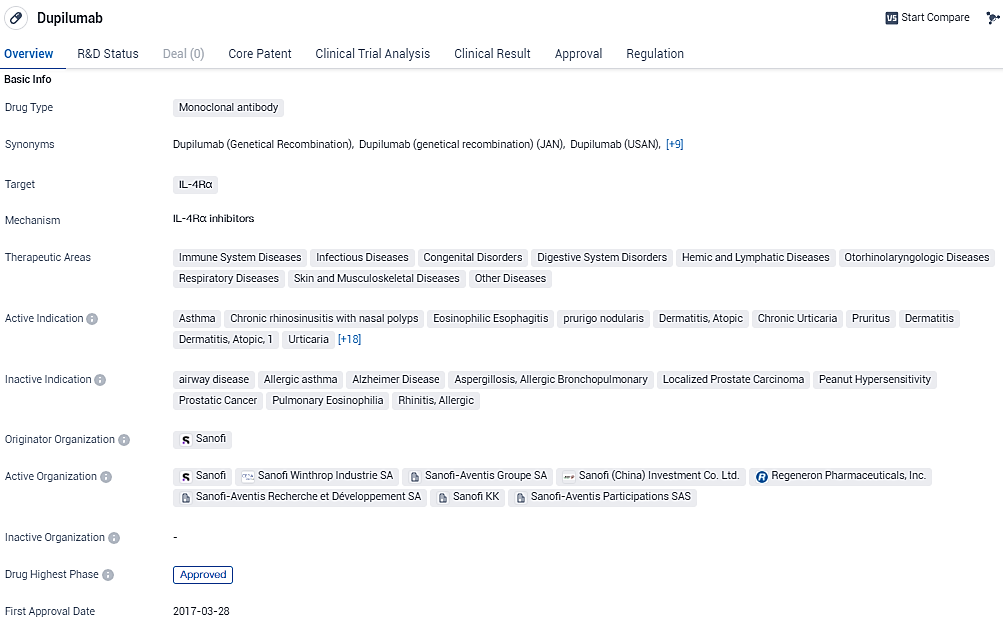
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The decision by the FDA to broaden the original EoE clearance from May 2022 now includes individuals as young as 1 year old who have a body weight of 15 kg or more. This encompassing authorization was expedited under the FDA's Priority Review category, which is dedicated to drugs that could offer considerable enhancements to treatment outcomes or safety for critical illnesses.
At its core, EoE is a persistent and advancing illness, fueled partly by type 2 inflammation, which causes harm to the esophagus and disrupts its normal operation. Children battling with EoE often face severe eating challenges and may suffer from symptoms such as acid reflux, emesis, discomfort in the abdomen, difficulties with swallowing, aversion to food, and growth delays.
These signs can be detrimental to their physical and developmental growth. Addressing EoE promptly and continually is crucial to mitigate potential troubles and the advance of the disease. It's estimated that around 21,000 U.S. children below 12 years old are receiving treatment for EoE with drugs that haven't been officially sanctioned for this use. Moreover, the actual number of affected children might be underreported due to misdiagnosed symptoms and diagnostic postponements.
As stated by Naimish Patel, M.D., who leads Global Development for Immunology and Inflammation at Sanofi, the sanction is indicative of their dedication to providing treatments for younger populations facing significant health gaps and provides a beacon of hope for these youngsters who are at a pivotal stage where consuming food and sustaining a healthy weight have direct correlations to their nutritional status and growth.
George D. Yancopoulos, M.D., Ph.D., currently co-chairing the board, who is also President and Chief Scientific Officer at Regeneron, indicated that with this new authorization, Dupixent stands as the sole treatment available for children aged 1 or older with a minimum body weight of 15 kg affected by EoE. By specifically addressing the type 2 inflammation that contributes to this ailment, Dupixent is poised to revolutionize the care paradigm for these youths just as it has with older juvenile and adult populations dealing with EoE.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
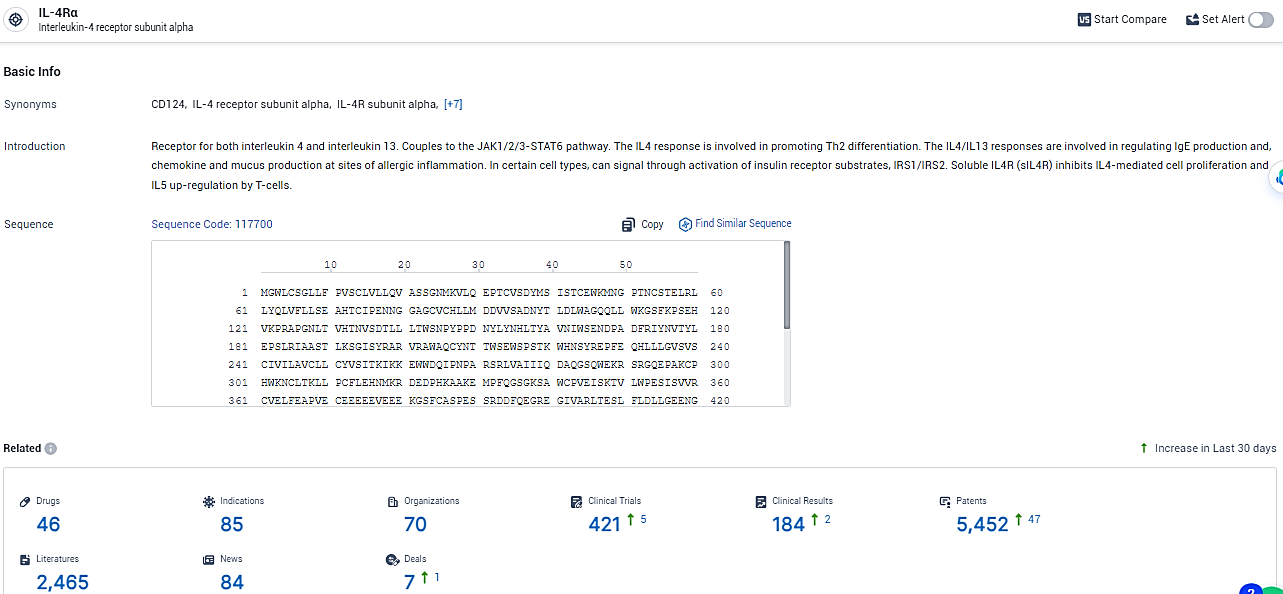
According to the data provided by the Synapse Database, As of January 31, 2024, there are 46 investigational drugs for the IL-4Rα target, including 85 indications, 70 R&D institutions involved, with related clinical trials reaching 421, and as many as 5452 patents.
Dupixent, which was invented using Regeneron's proprietary VelocImmune® technology, is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways and is not an immunosuppressant. Dupilumab represents an important advancement in the field of biomedicine. As an expert in the pharmaceutical industry, it is crucial to closely monitor the developments and advancements related to Dupilumab, as it has the potential to significantly impact patient care and contribute to the treatment of various diseases.