Electra Therapeutics Unveils Phase 1b Trial Results of ELA026 for sHLH at EHA2024
Electra Therapeutics, Inc., a biotechnology company in the clinical stage, focusing on developing antibody therapies targeting new factors in immunological disorders and cancer, revealed clinical data regarding ELA026 for secondary hemophagocytic lymphohistiocytosis, a severe hyperinflammatory condition.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Outcomes have been presented for sHLH patients enrolled in the currently ongoing Phase 1b trial, focusing predominantly on individuals with the most lethal sHLH variant, malignancy-associated HLH. For treatment-naive mHLH patients, ELA026 achieved a complete response rate of 100% by the fourth week and also demonstrated enhanced survival rates at the two-month mark in comparison to historical data. ELA026 showed high efficacy across a broad spectrum of sHLH patients and maintained a favorable safety profile.
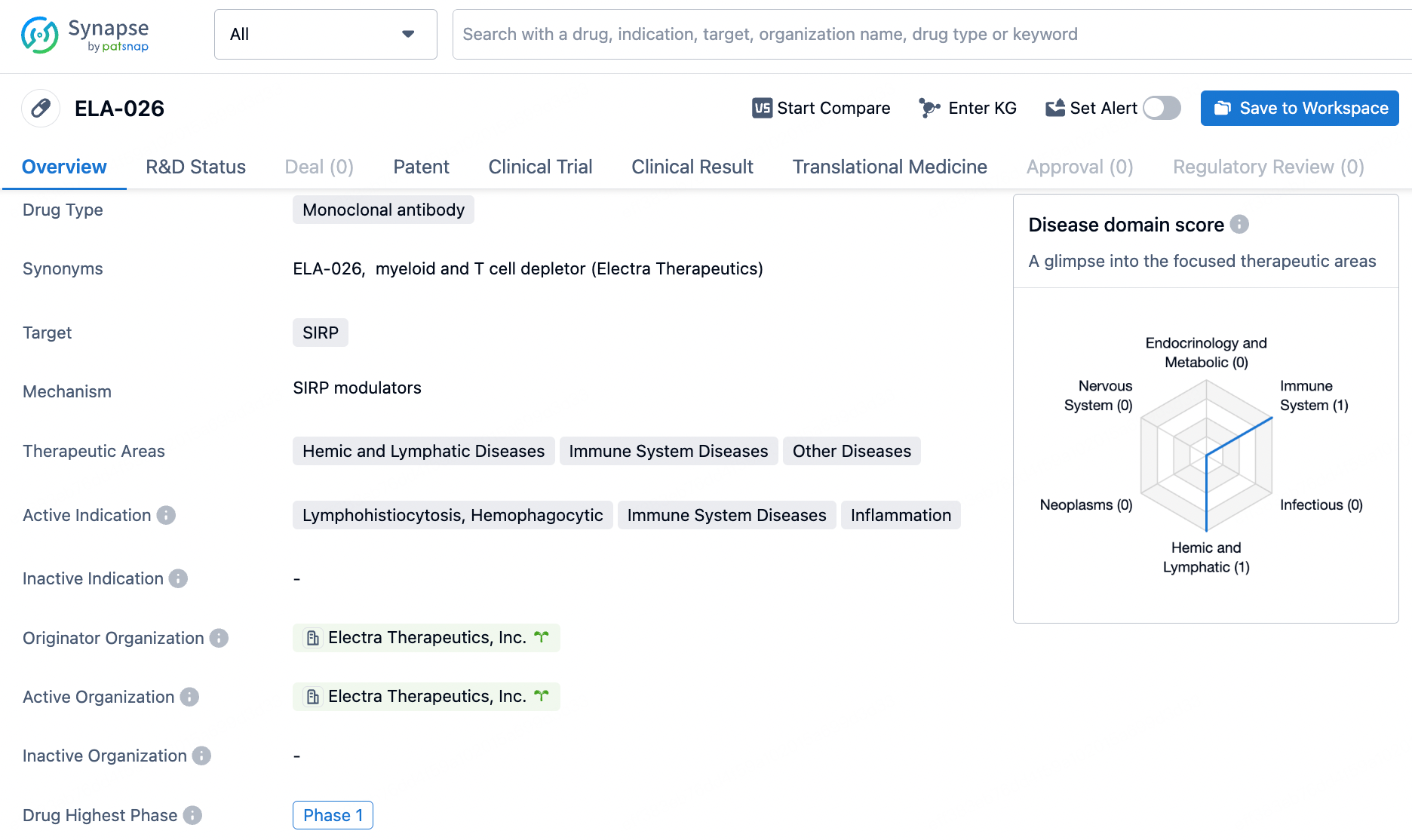
ELA026 is an innovative monoclonal antibody that targets signal regulatory protein (SIRP)-α/β1/γ expressed on the surface of myeloid cells and T lymphocytes, which are the pathological immune cells triggering hyperinflammation in sHLH. This Phase 1b trial is an ongoing open-label, multi-dose, single-arm, multicenter study aimed at evaluating the safety and efficacy of ELA026, examining biomarkers, and determining a suitable dose for later Phase 2/3 studies.
"These findings indicate highly encouraging outcomes for ELA026 as a potential treatment for sHLH, a severe disease with devastating impacts on patients and no approved treatments," said Swaminathan P. Iyer, MD, Professor in the Lymphoma/Myeloma Department at The University of Texas MD Anderson Cancer Center. "Importantly, the analysis shows that ELA026 led to improved survival in treatment-naive mHLH patients, highlighting the benefits of early intervention in this rapidly progressing condition," added Swaminathan P. Iyer.
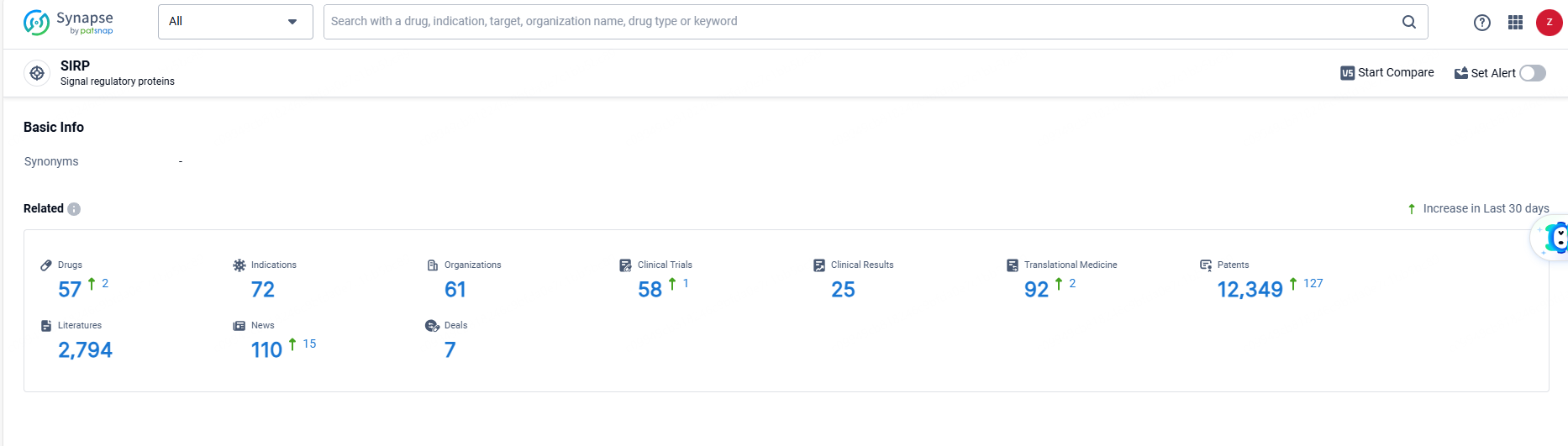
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 19, 2024, there are 57 investigational drugs for the SIRP targets, including 72 indications, 61 R&D institutions involved, with related clinical trials reaching 58, and as many as 12349 patents.
ELA-026 is a monoclonal antibody drug targeting SIRP and being developed for the treatment of hemic and lymphatic diseases, immune system diseases, and other conditions. With its active indications including lymphohistiocytosis, hemophagocytic, immune system diseases, and inflammation, ELA-026 has the potential to address unmet medical needs in these therapeutic areas. As it progresses through clinical development, Electra Therapeutics, Inc. will continue to advance ELA-026 towards potential approval.