Promising Outcomes for Neurona's NRTX-1001 in Resistant Epilepsy Revealed at 2024 AAN
Neurona Therapeutics, a firm in the clinical-phase specializing in biotherapeutic developments aimed at regenerative cell treatments for neurological conditions, is set to share new preliminary results from its continuous Phase 1/2 open-label study of NRTX-1001. This study involves adults suffering from drug-resistant mesial temporal lobe epilepsy on one side and will be discussed at the 2024 Annual Meeting of the American Academy of Neurology in Denver, CO.
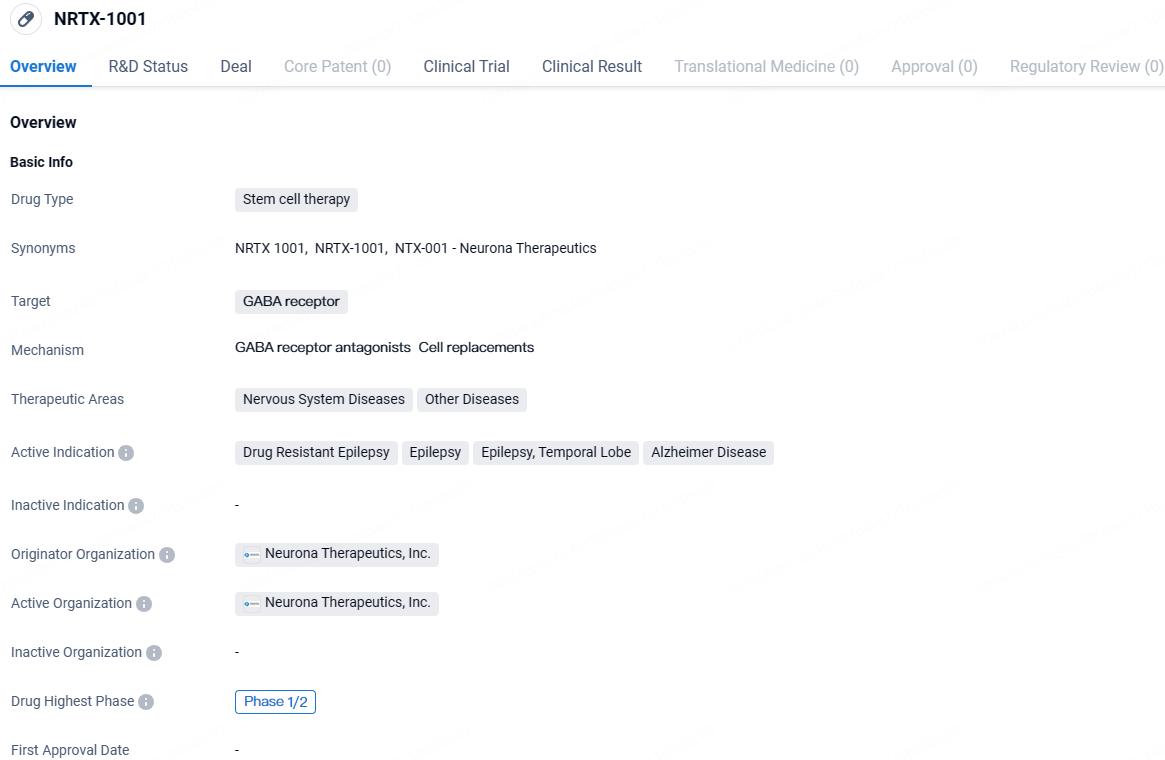
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In an initial group comprising five participants treated with a smaller dosage of NRTX-1001, results showed a typical decrease in seizure frequency of 75% from their original levels over a six-month period post-treatment.
Among these participants, four experienced a reduction in seizures exceeding 50%. Two individuals from this group have been monitored for durations of 16 and 21 months after receiving NRTX-1001, consistently indicating a sustained decrease in seizure occurrences by over 95%, alongside enhancements in quality-of-life assessments. To this point, NRTX-1001 has been tolerated without issues by everyone involved, and there has been no evidence of neurocognitive deficits as per neuropsychological evaluations.
John Hixson, M.D., the senior medical director at Neurona, expressed optimism about the outcomes, noting the significant and ongoing control of seizures seen in these first participants, which continues for up to 21 months after the initial administration. Dr. Hixson highlighted that unlike lobectomy, a surgical procedure with potential cognitive repercussions from brain tissue removal, NRTX-1001 offers a cell therapy alternative designed to achieve neural balance while preserving cognitive capabilities.
So far, NRTX-1001 has been accepted well by all participants. Only one individual from the initial batch experienced severe adverse effects in the form of a seizure cluster and a related kidney issue in the third month following treatment, which were linked to the person's medical background rather than the treatment or related procedures.
Other recorded adverse effects have been mild or moderate, largely associated with the delivery method or the immunosuppressive treatment, all of which subsided within the initial month or following the cessation of immunosuppression as per the procedure outlined in the second year.
The first pair from another ongoing trial cohort has been administered a greater dose of NRTX-1001 and have not encountered any significant adverse effects thus far.
Additionally, the FDA’s recent approval of a second trial to assess NRTX-1001 on 10 patients with drug-resistant bilateral MTLE is a significant development. Recruitment for this study is active at several epilepsy centers nationwide.
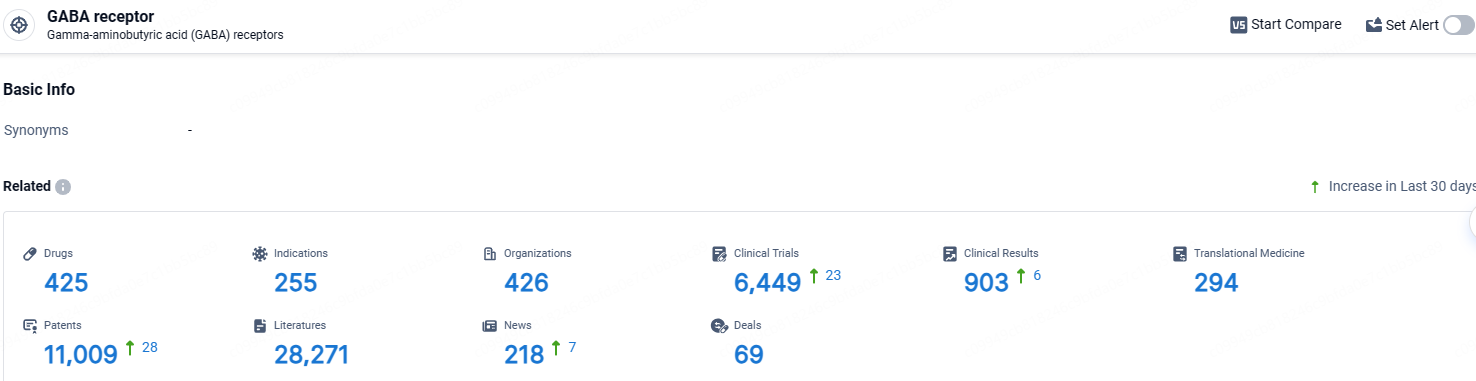
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 18, 2024, there are 425 investigational drugs for the GABA receptor, including 255 indications, 426 R&D institutions involved, with related clinical trials reaching 6449, and as many as 294 patents.
Stem cell therapy is a promising field in biomedicine, offering potential solutions for various diseases and conditions. By targeting the GABA receptor, NRTX-1001 aims to modulate the inhibitory neurotransmitter system in the brain, which plays a crucial role in regulating neuronal activity. This approach holds promise for addressing the underlying causes of epilepsy and Alzheimer's disease.