Elpiscience and Astellas Pharma have formed a new R&D and licensing partnership to create innovative dual-targeting macrophage engagers
Elpiscience Biopharma, Ltd., Limited has entered into a partnership with Astellas Pharma Incorporated to undertake joint research initiatives and secure licensing contracts concerning innovative dual-specificity macrophage engagement agents, specifically ES019 and an additional project. Both firms will jointly engage in preliminary research activities associated with these two distinct ventures.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Elpiscience has agreed to provide Astellas with the opportunity to incorporate up to two more initiatives into their joint efforts. Should Astellas decide to pursue these additional options, Elpiscience is committed to allotting exclusive access for Astellas to continue with the exploration, progression, production, and sales endeavors for each individual initiative.
As a biopharmaceutical enterprise still in its clinical phase and not yet publicly traded, Elpiscience concentrates on forging innovative treatments in the realm of immuno-oncology to aid cancer sufferers across the globe. At the heart of their research lies the Bispecific Macrophage Engager Platform, a potent construct featuring dual-focused antibodies targeting tumor-specific antigens alongside the signal-regulatory protein alpha (SIRPα). The purpose is to stimulate Tumor Associated Macrophage (TAM) phagocytosis that specifically destroys tumor cells expressing target antigens.
The effectiveness of the BiME platform stems from its capacity to prompt phagocytosis in TAMs by engaging the Fc receptors on them and through simultaneous interaction with both TAA and SIRPα on tumor cells, while also inhibiting the CD47-SIRPα "don't eat me" signal. One of the promising products devised from this platform is ES019, an intricate anti-PD-L1/SIRPα bispecific antibody.
Within various cancer types, TAMs predominate the Tumor Microenvironment (TME) and are associated with negative clinical outcomes and resistance to immune checkpoint blockers. Breakthroughs from the BiME initiative herald the possibility of introducing innovative treatments for those cancer patients for whom current immunotherapies have failed, functioning by altering TAM behavior and transforming the condition of the TME.
Elpiscience is set to receive payments adding up to $37 million, inclusive of initial compensation and fees for licensing options. Alongside this, Astellas will supply research funding to propel the initiatives forward. Upon option execution by Astellas, Elpiscience stands to obtain over $1.7 billion tied to the attainment of future developmental, regulatory, and sales milestones. Additionally, based on the net sales of approved products from each project, Elpiscience will earn royalty payments ranging from the single digits to the lower teens in percentage terms.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
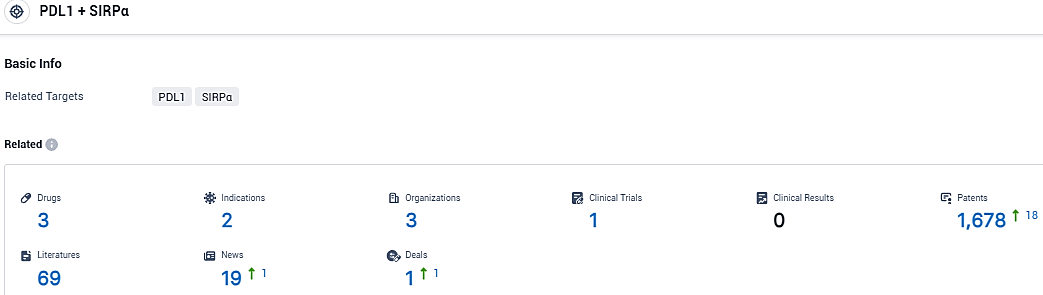
According to the data provided by the Synapse Database, As of January 04, 2024, there are 3 investigational drugs for the PD-L1/SIRPα target, including 2 indications, 3 R&D institutions involved, with related clinical trials reaching 1, and as many as 1678 patents.
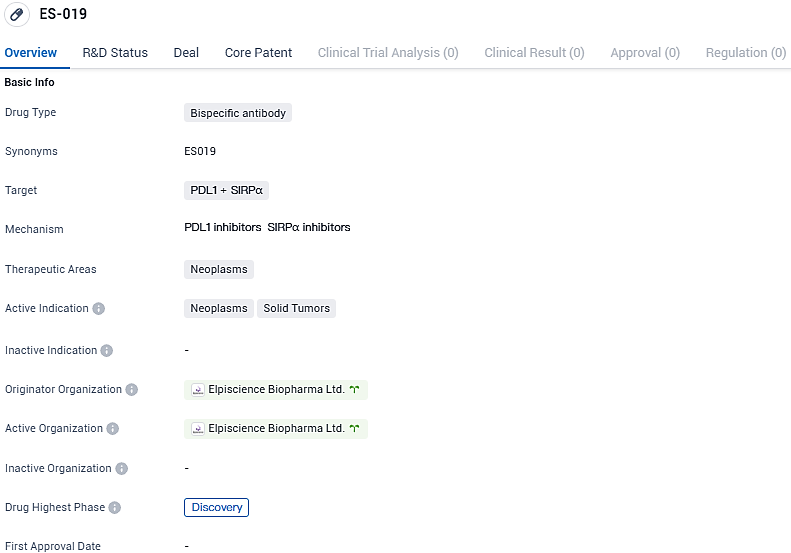
ES019 is a bispecific antibody that targets PDL1 and SIRPα in the therapeutic area of neoplasms, specifically solid tumors. It is currently in the discovery phase of development, both globally and in China, and is being developed by Elpiscience Biopharma Ltd. Further research and development will be needed to determine the potential efficacy and safety of ES019 in the treatment of neoplasms.