Endostar: Brief Review of its R&D progress and the clinical result in 2023 ESMO
On 23 Oct 2023, the results from a phase II trial of endostar in patients with locally advanced squamous cell carcinoma of esophagus were reported at the ESMO Congress.
Endostar's R&D Progress
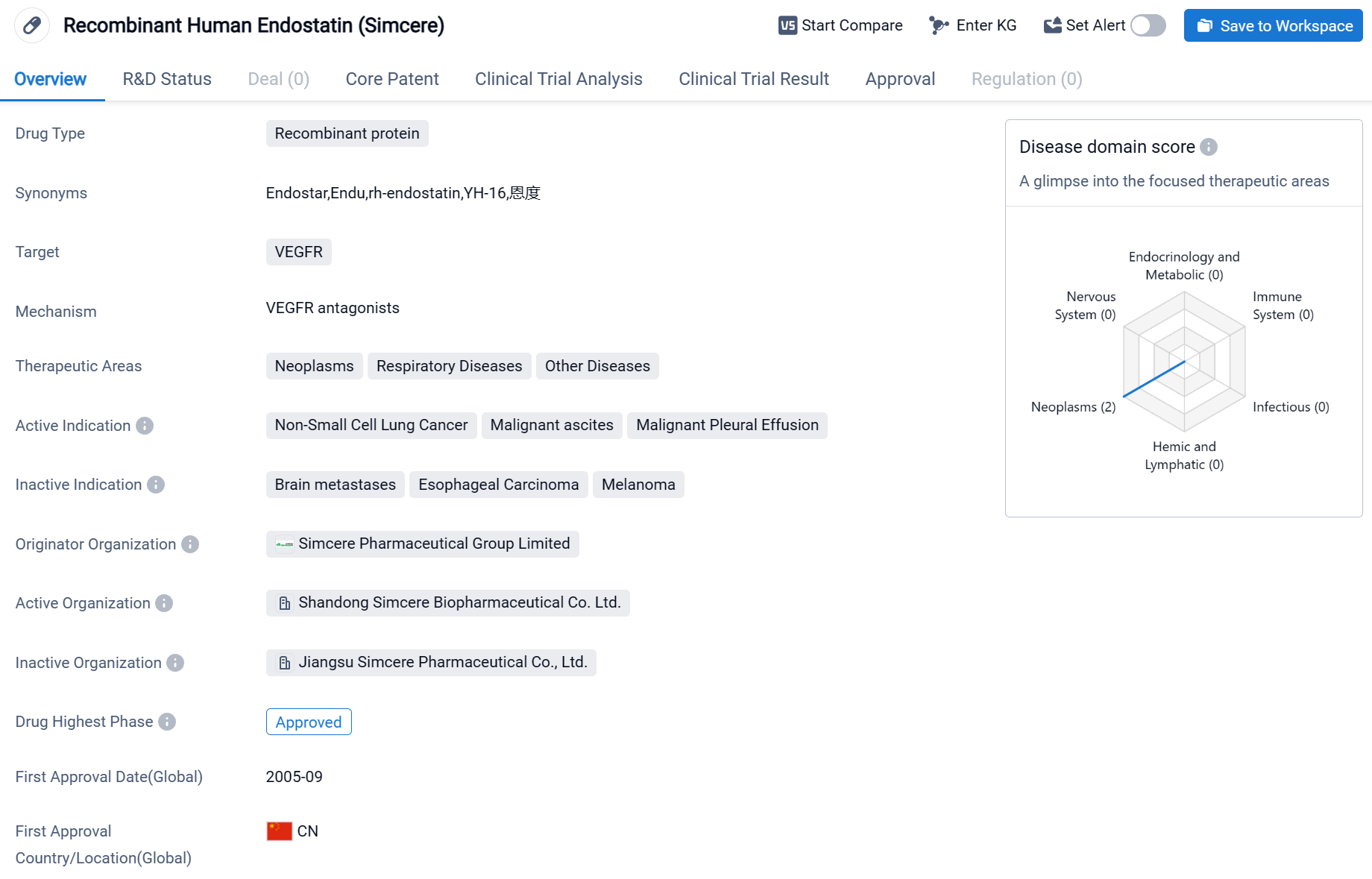
Endostar is a drug classified as a recombinant protein and is primarily used in the field of biomedicine. It targets VEGFR, which stands for vascular endothelial growth factor receptor. This drug is indicated for the treatment of various conditions, including neoplasms (abnormal growth of cells), respiratory diseases, and other diseases.
According to the Patsnap Synapse, Endostar has reached the highest phase of approval globally. And the clinical trial areas for Endostar are primarily in the China and United States. The key indication is Non-Small Cell Lung Cancer. 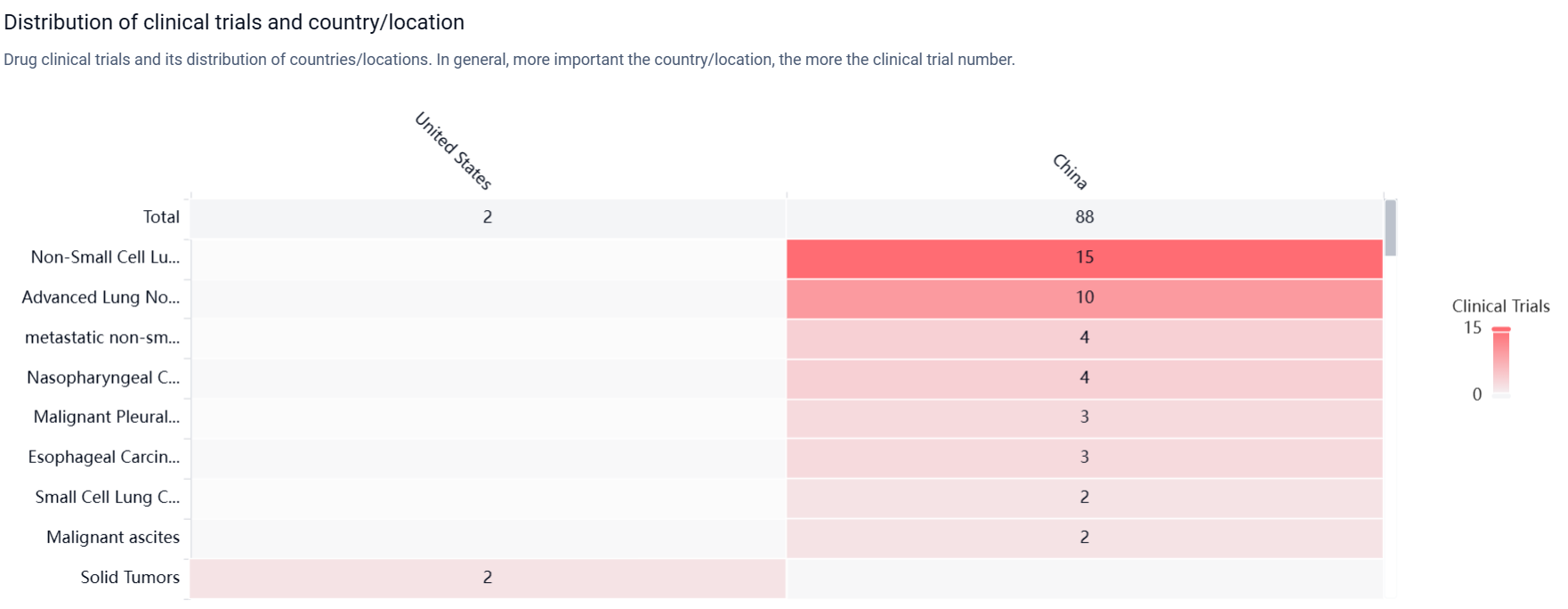
Detailed Clinical Result of Endostar
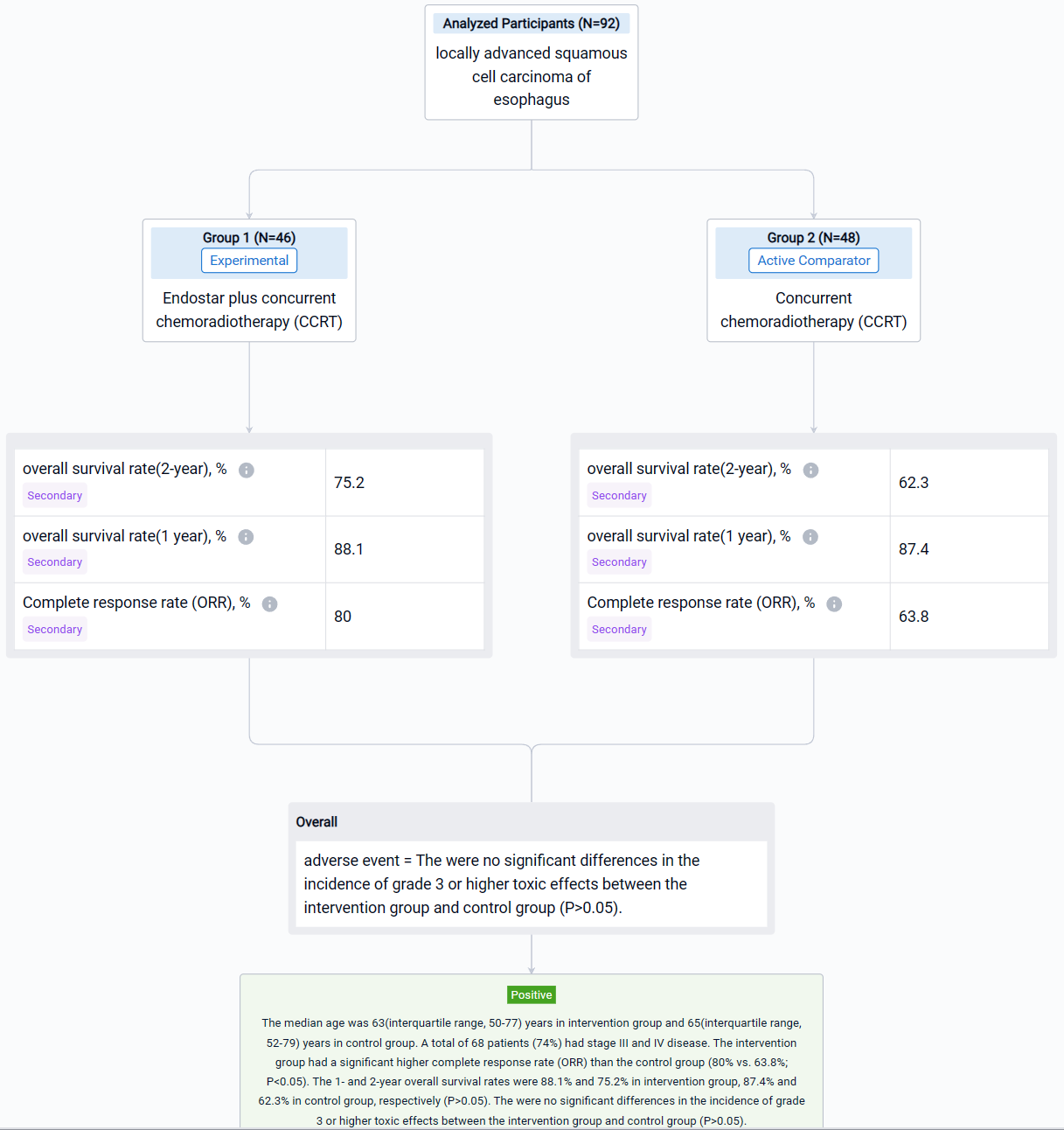
This phase II trial was to evaluate the efficacy and safety of Endostar combined with concurrent chemoradiotherapy (CCRT) in patients with locally advanced esophageal squamous cell carcinoma (ESCC).

In this study, 92 patients with unresectable thoracic ESCC, clinical staged as IB to IVB disease based on the 8th edition of the American Joint Committee on Cancer (stage IVB: Only metastasis to supraclavicular/celiac lymph nodes) and local recurrence, were enrolled and randomly allocated in Endostar plus concurrent chemoradiotherapy (CCRT)(intervention group; n=46) and concurrent chemoradiotherapy (CCRT) (control group; n=48). Five or six cycles of Endostar (7.5mg/m2/24h ×120h, 7 days/cycle) were delivered in intervention group. The primary endpoint was overall survival (OS). The secondary endpoints were progression-free survival (PFS), 1-year and 2-year overall survival rate and adverse events (AE).
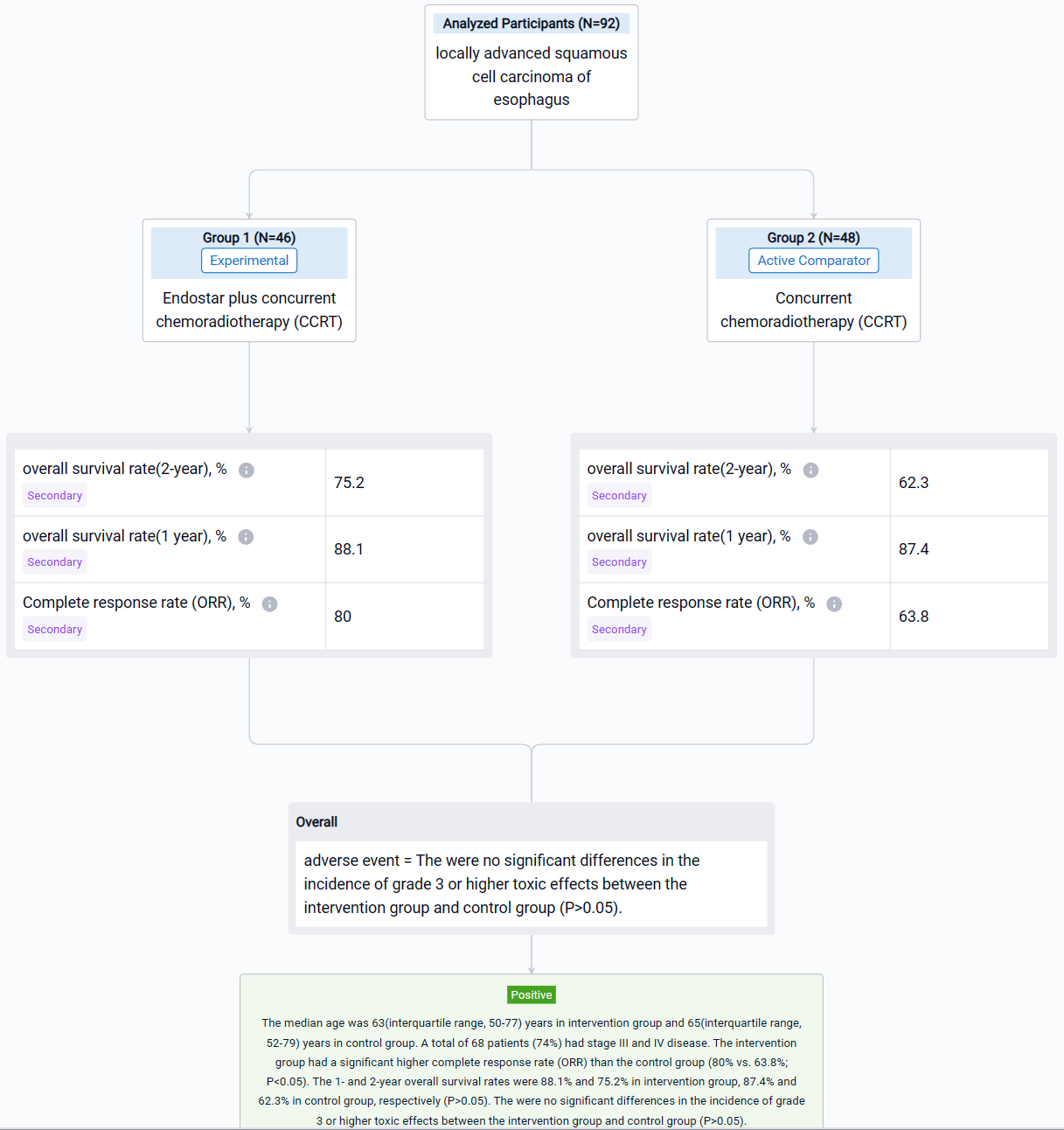
The result showed that the intervention group had a significant higher complete response rate (ORR) than the control group (80% vs. 63.8%; P<0.05). The 1- and 2-year overall survival rates were 88.1% and 75.2% in intervention group, 87.4% and 62.3% in control group, respectively (P>0.05). The were no significant differences in the incidence of grade 3 or higher toxic effects between the intervention group and control group (P>0.05).
It can be concluded that Endostar plus concurrent chemoradiotherapy has better efficacy and safety for patients with locally advanced esophageal squamous cell carcinoma.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
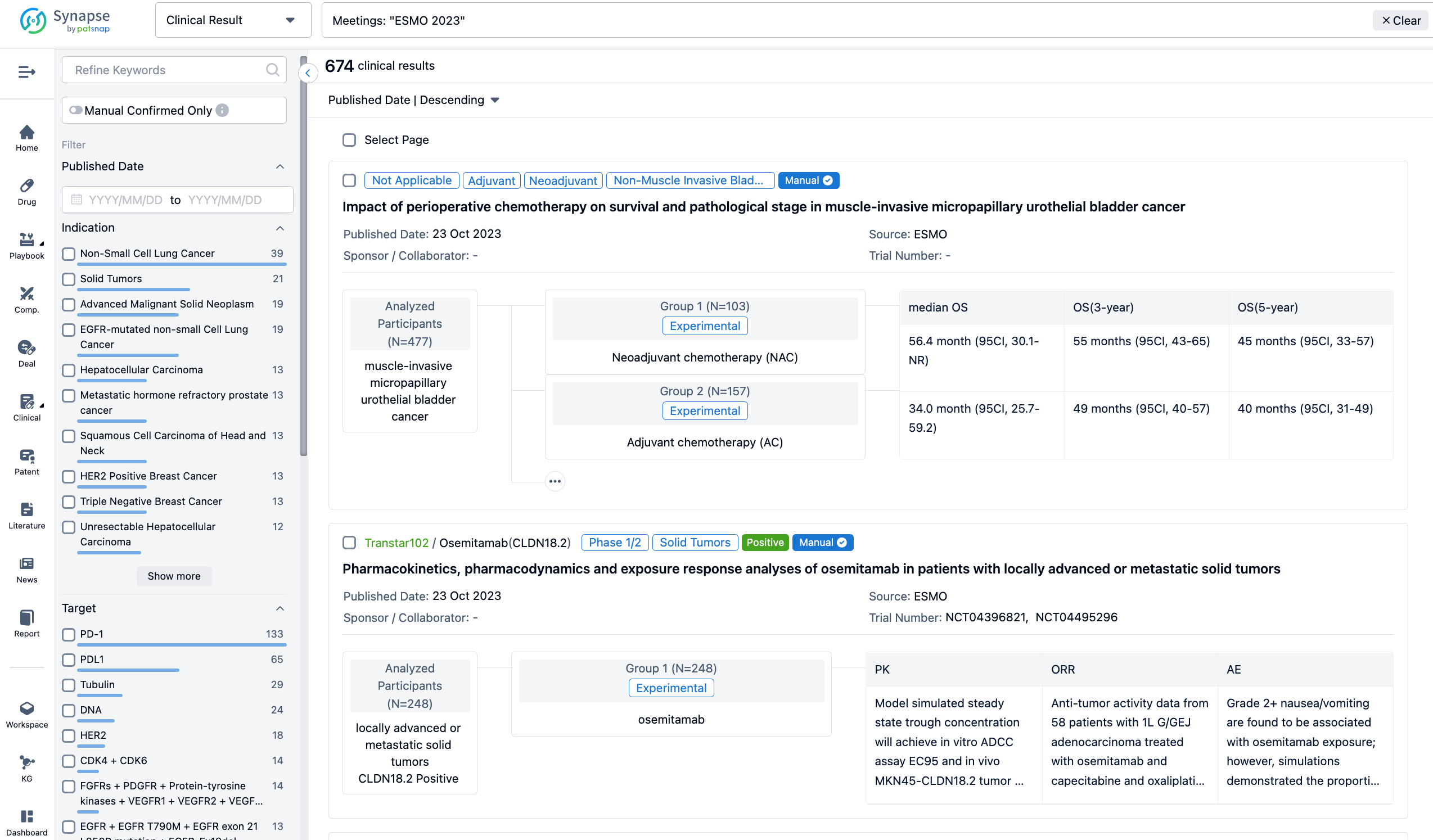
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

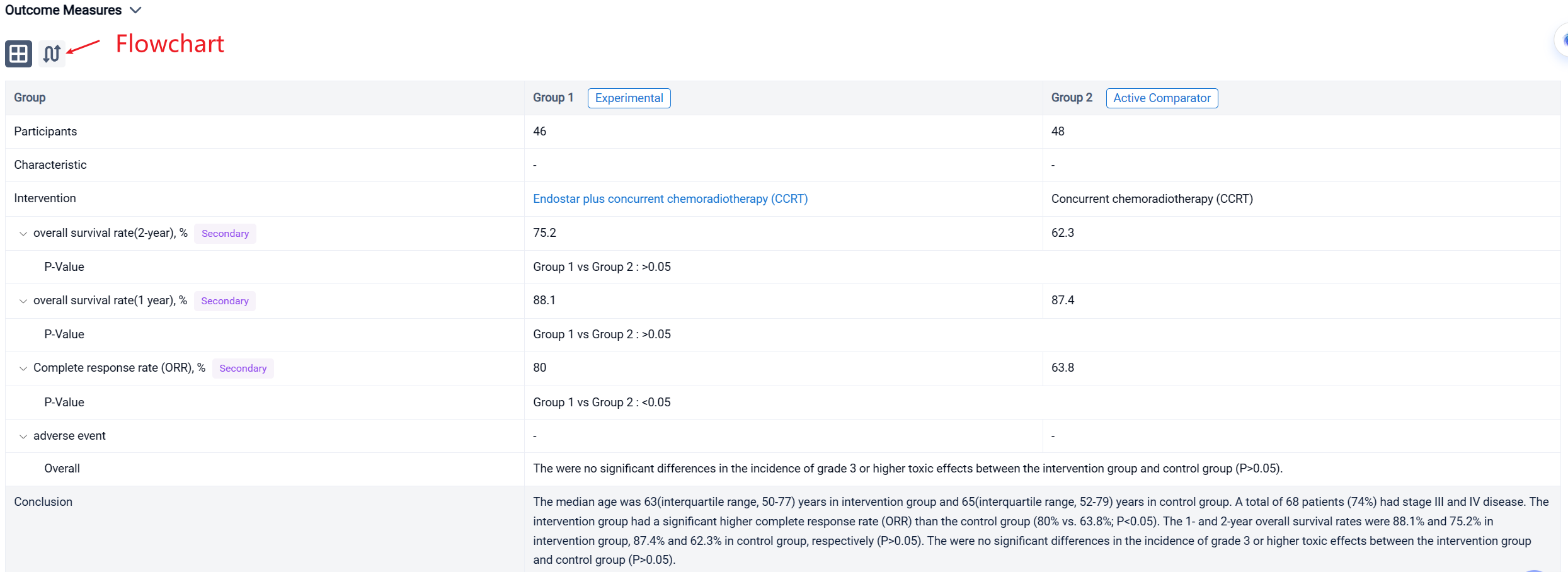
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
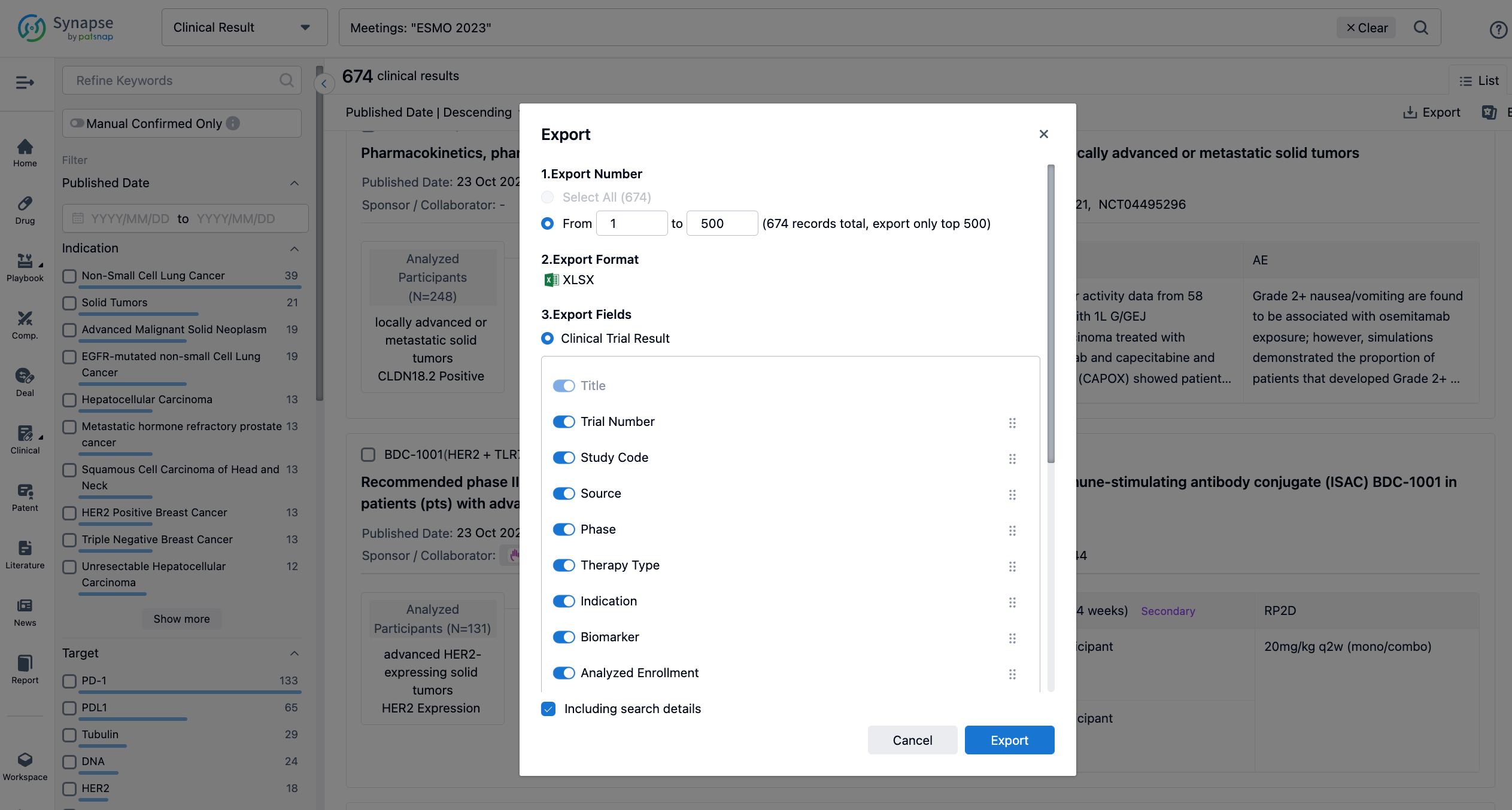
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!