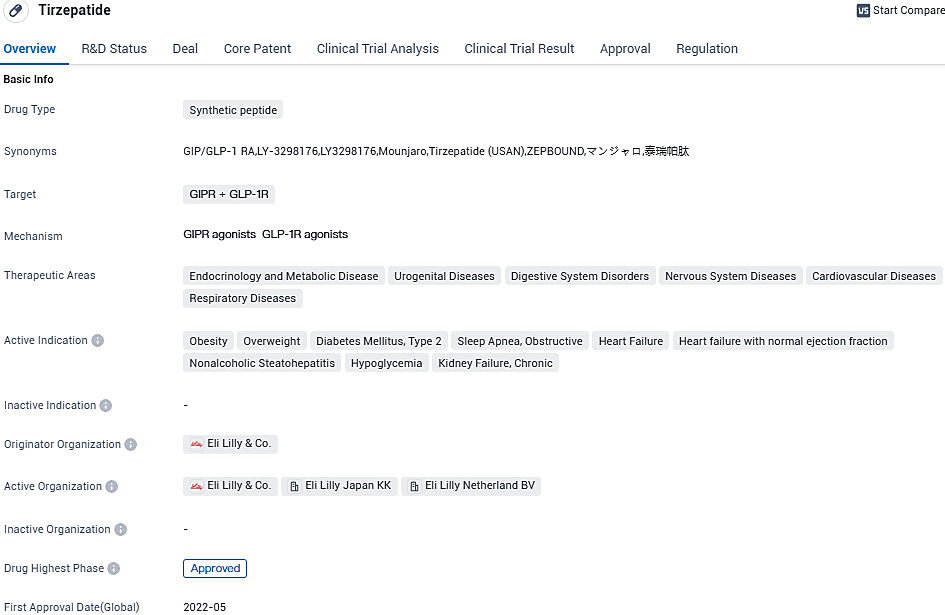
The FDA approves Lilly's Zepbound™ (tirzepatide) - an effective new option for managing continual weight issues such as obesity or health-related overweight conditions
The FDA has given its approval to Eli Lilly's Zepbound™ (tirzepatide) injection, a unique obesity treatment that stimulates both GIP and GLP-1 hormone receptors, making it the first of its kind. Zepbound is intended for use in adults struggling with obesity or those who, despite being overweight, are also experiencing health complications related to their weight like hypertension, dyslipidemia, type 2 diabetes mellitus, obstructive sleep apnea, or cardiovascular disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Utilizing Zepbound in conjunction with any other tirzepatide-based medications or medicines that contain GLP-1 receptor agonist is strongly discouraged. Also, no research has taken place to study its effects on individuals with a past record of pancreatitis or severe gastrointestinal diseases, such as extreme gastroparesis.
Joe Nadglowski, who is the head honcho and CEO of Obesity Action Coalition pointed out, "Despite being aware that obesity is a controllable, long-lasting health condition, people struggling with it still encounter many obstacles in their path towards health and weight control. The introduction of new treatments provides a beacon of hope to those suffering from obesity and seeking improved weight control methods."
Zepbound was not sanctioned to cure these conditions but in a controlled study, participants who lead a healthy lifestyle in terms of diet and workout and simultaneously took Zepbound to regulate their obesity or overweight related health issues, experienced alterations in their cholesterol levels with a decrease in their waist circumference and blood pressure.
Zepbound™ (tirzepatide), an injection that has received the approval of the FDA, is often used as an auxiliary to a low-calorie diet regime and amplified physical activity for the long-term control of weight in adults dealing with obesity who also have at least one weight-related concurrent condition. It's noteworthy that Zepbound is the single FDA endorsed obesity treatment that triggers both GIP and GLP-1 hormone receptors.
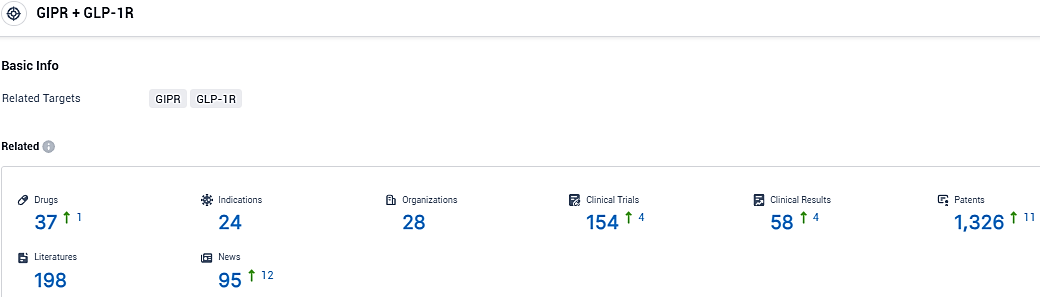
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 14, 2023, there are 37 investigational drugs for the GIPR and GLP-1R target, including 24 indications, 28 R&D institutions involved, with related clinical trials reaching 154, and as many as 1326 patents.
Tirzepatide is indicated for various conditions such as obesity, diabetes, heart failure, and chronic kidney failure. With its recent approval in the United States and ongoing regulatory process in China, Tirzepatide shows promise in addressing multiple therapeutic areas and may have a significant impact on patients' lives.