Entera Bio Reveals Promising Oral GLP-2 Pill Efficacy for Short Gut Syndrome
Entera Bio Ltd. a pioneer in creating peptide treatments that can be administered orally, has revealed encouraging findings related to drug dynamics from a joint study project. This research involves integrating a unique, extended-duration GLP-2 agonist from OPKO Health, Inc. with Entera's exclusive N-Tab™ platform.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This initiative is aimed at creating a pioneering GLP-2 peptide-based tablet as a new therapeutic option for individuals afflicted with short bowel syndrome and other conditions characterized by mucosal inflammation and challenges with nutrient absorption.
Gattex (teduglutide) and the sole sanctioned GLP-2 agonist, necessitates a daily subcutaneous injection for administration. Partners Zealand Pharma and Ironwood Pharmaceuticals are in the process of innovating GLP-2 treatments that offer extended duration, reducing the frequency to once or twice per week for injections.
Entera Bio Ltd. in collaboration with OPKO Health Inc. has successfully carried out a single-dose pharmacokinetic exploration in rodent models, which serves as the inaugural benchmark for the feasibility of delivering the GLP-2 therapy orally. The trial involved administering either oral tablets or intravenous injections of the medication to rats and monitoring pharmacokinetics through blood sampling over the course of a day post-administration, with the resulting drug concentrations being measured utilizing an established LC-MS/MS protocol.
The goals of the investigation were achieved, with the orally delivered GLP-2 tablets showing substantial systemic absorption. Moreover, it was observed that the plasma concentration levels attained with this novel oral format of the GLP-2 analog were significantly higher, by a magnitude of approximately 10 times, than the therapeutic plasma levels documented for Gattex when given subcutaneously, as specified on the drug's label.
Further analysis of the pharmacokinetic data derived from the intravenous application of the GLP-2 peptide disclosed that the peptide's plasma half-life in rats extended up to six times longer than teduglutide’s half-life in the analogous rodent model. This finding aligns with existing pharmacokinetic data about OPKO’s GLP-2 peptide, famed for its prolonged action and previously crafted for weekly subcutaneous dosing.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
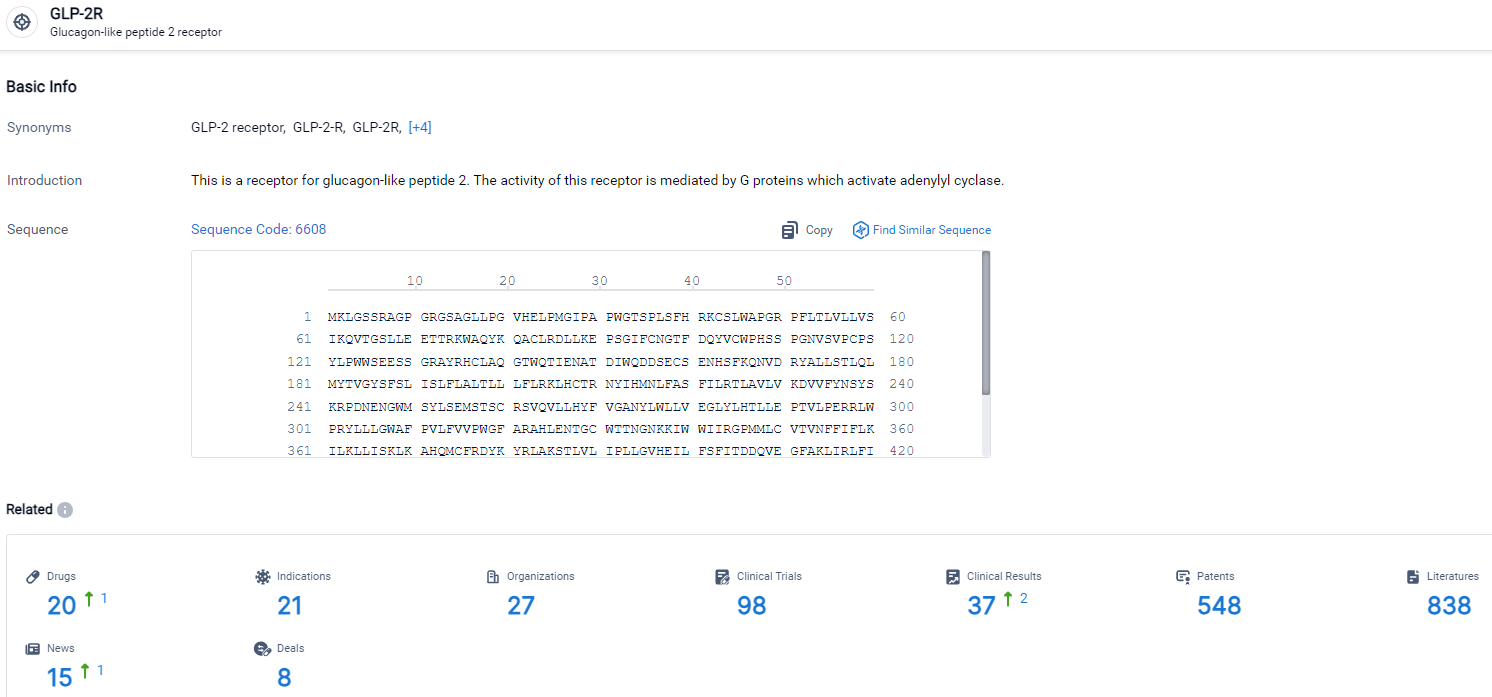
According to the data provided by the Synapse Database, As of March 22 2024, there are 20 investigational drugs for the GLP-2R target, including 21 indications, 27 R&D institutions involved, with related clinical trials reaching 98, and as many as 838 patents.
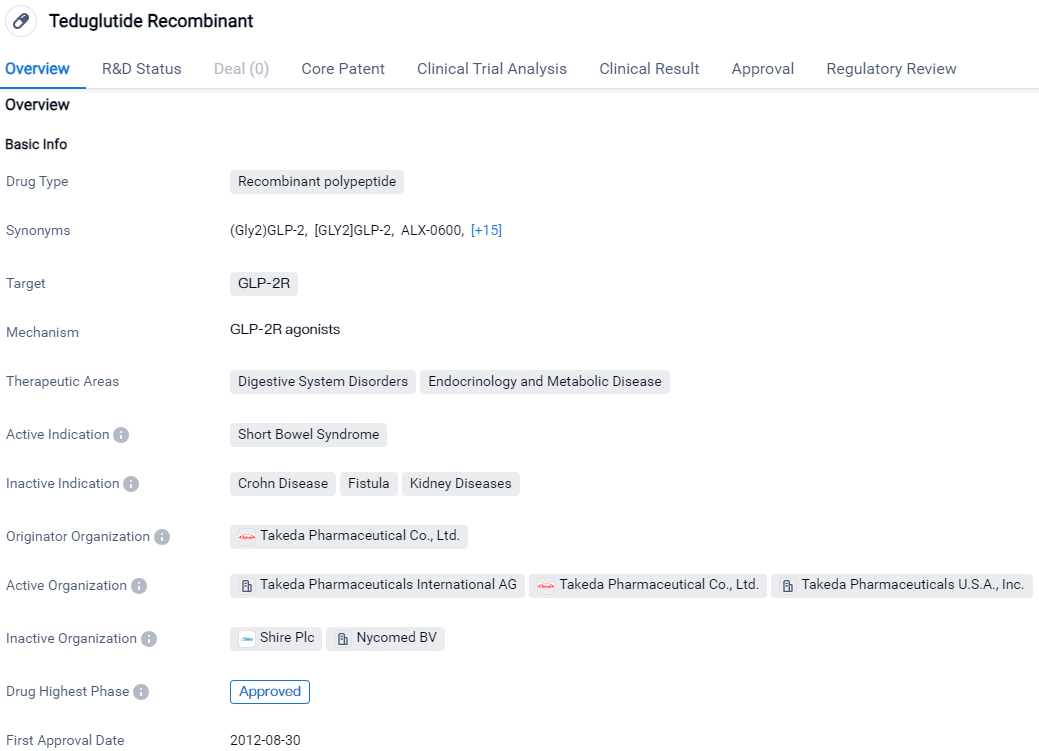
Teduglutide Recombinant is a recombinant polypeptide drug that targets the GLP-2R and is primarily used for the treatment of Short Bowel Syndrome. It has been approved globally and in China, with the first approvals occurring in 2012 in the European Union, Iceland, Liechtenstein, and Norway. The drug is classified as an orphan drug, indicating its focus on treating a rare condition.