EU Commission Approves Expanded Use of Astellas' XTANDI™ for Early-Stage Recurrent Prostate Cancer
Astellas Pharma Inc. disclosed that the European Commission has granted approval for an expanded use of XTANDI™ (enzalutamide), either as a standalone treatment or paired with androgen deprivation therapy, aimed at managing high-risk biochemical recurrent non-metastatic hormone-sensitive prostate cancer in adult males who are not suitable candidates for salvage-radiotherapy.
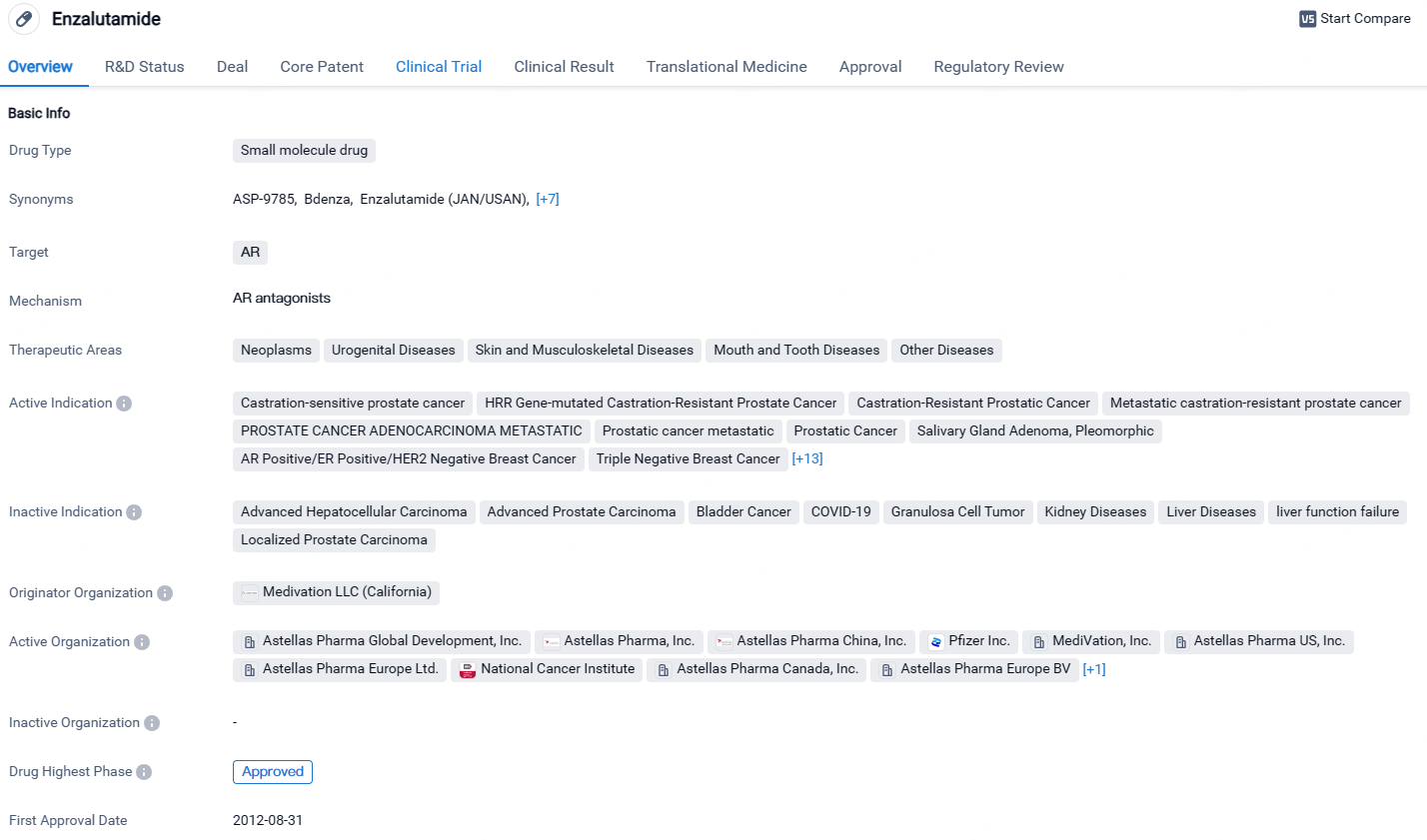
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The expanded authorization of XTANDI follows research from the EMBARK Phase 3 study, which involved 1,068 male participants with high-risk BCR nmHSPC. In these participants, prostate-specific antigen levels, a marker potentially indicative of prostate cancer, had doubled within a period of nine months or less.
The data revealed that individuals receiving a combination therapy of XTANDI and leuprolide witnessed a 57.6% decrease in the likelihood of their cancer progressing or leading to death compared to those on leuprolide alone. Additionally, using XTANDI as a monotherapy resulted in a 36.9% reduction in the risk of cancer progression or death.
In April 2024, the European Association of Urology updated their guidelines to endorse the use of enzalutamide for men diagnosed with high-risk BCR nmHSPC, applicable both during and post-radiation therapy or surgery, regardless of ADT usage. This revision came as previously there was no agreed-upon standard care protocol for this group.
Following a favorable review in March 2024 by the Committee for Medicinal Products for Human Use at the European Medicines Agency, XTANDI received endorsement for use in men with high-risk BCR nmHSPC from the European Commission.
In the United States, the Food and Drug Administration granted approval for XTANDI in November 2023 for treating patients with non-metastatic castration-sensitive prostate cancer at high risk of metastasis. Astellas has indicated that it will update its fiscal projections for the year ending March 31, 2025, to include this development, with a formal announcement to be made on April 25, 2024.
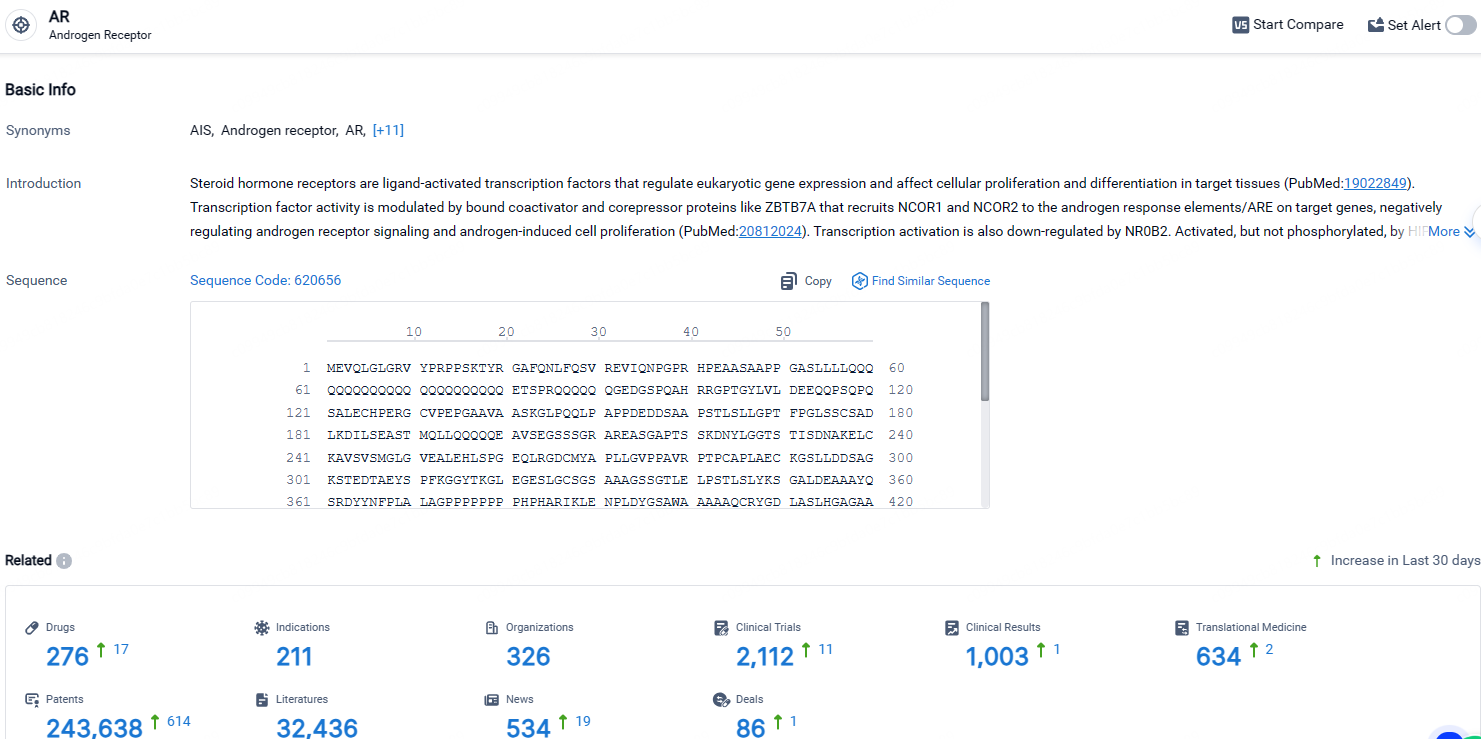
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 26, 2024, there are 276 investigational drugs for the AR target, including 211 indications, 326 R&D institutions involved, with related clinical trials reaching 2112, and as many as 243638 patents.
Enzalutamide is a small molecule drug that targets the androgen receptor. It has been approved for various indications, primarily related to prostate cancer and breast cancer. The drug has obtained global and Chinese approvals. Its first approval was granted in the United States in 2012. The drug has undergone regulatory processes such as priority review and fast track, highlighting its potential to address critical medical needs.