hC Bioscience Unveils Hemophilia Project and Promising Results from tRNA Anticodon Strategy
At the 2024 World Congress of the World Federation of Hemophilia in Madrid, Spain, hC Bioscience, a biopharmaceutical firm focused on pioneering a revolutionary tRNA-based protein editing technology for treating genetic disorders, disclosed preclinical findings that bolster its primary initiative for addressing severe hemophilia A.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Jose Lora, Ph.D., the Chief Science Officer at hC Bioscience, has announced the introduction of their initial development candidate, HCB-101. This anticodon engineered tRNA is crafted to counteract the effects of nonsense mutations. The delivery system for HCB-101 utilizes lipid nanoparticles to specifically target the liver, the site of Factor VIII synthesis.
The presented research indicates that HCB-101 effectively reaches the liver in mouse models and enables the in vitro synthesis of a complete, active form of Factor VIII, despite the presence of a premature termination codon that normally leads to a nonfunctional, truncated protein. This technique shows promise for treating approximately 20% of severe hemophilia A cases and potentially a wide range of other conditions linked to nonsense mutations.
Leslie Williams, CEO of hC Bioscience, expressed enthusiasm about the prospects of tRNA-based protein editing to provide a pioneering treatment for severe hemophilia A. "Our primary project is proceeding smoothly towards clinical trials, aiming to validate this innovative protein editing strategy as a flexible solution for various genetic diseases," she stated.
Williams further commented, “Our treatments work irrespective of the gene involved. The same engineered tRNA that bypasses the premature termination codon in hemophilia can also interpret premature termination codons in numerous other genetic disorders. We envision tRNA not merely as a new tool but as a robust, universal approach that amplifies the capabilities of genomic medicine to enhance patient outcomes."
hC Bioscience is set to advance from these preclinical findings to studies that are necessary for Investigational New Drug (IND) applications, with plans to initiate a Phase 1 clinical trial for severe hemophilia A by 2025. Concurrently, the company plans to organize a clinical advisory board meeting at the World Federation of Hemophilia 2024 World Congress.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
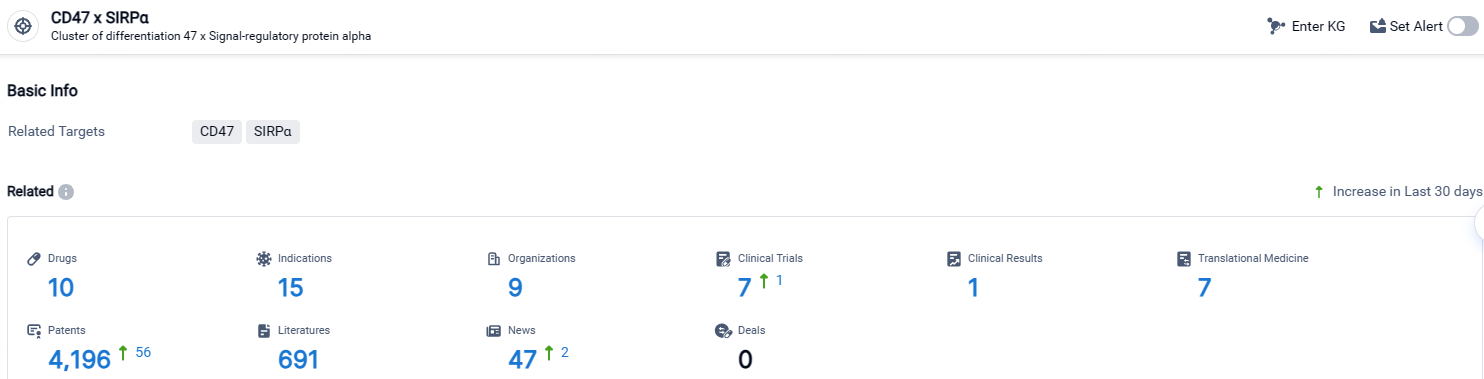
According to the data provided by the Synapse Database, As of April 25, 2024, there are 10 investigational drugs for the CD47 x SIRPα target, including 15 indications, 9 R&D institutions involved, with related clinical trials reaching 7, and as many as 4196 patents.
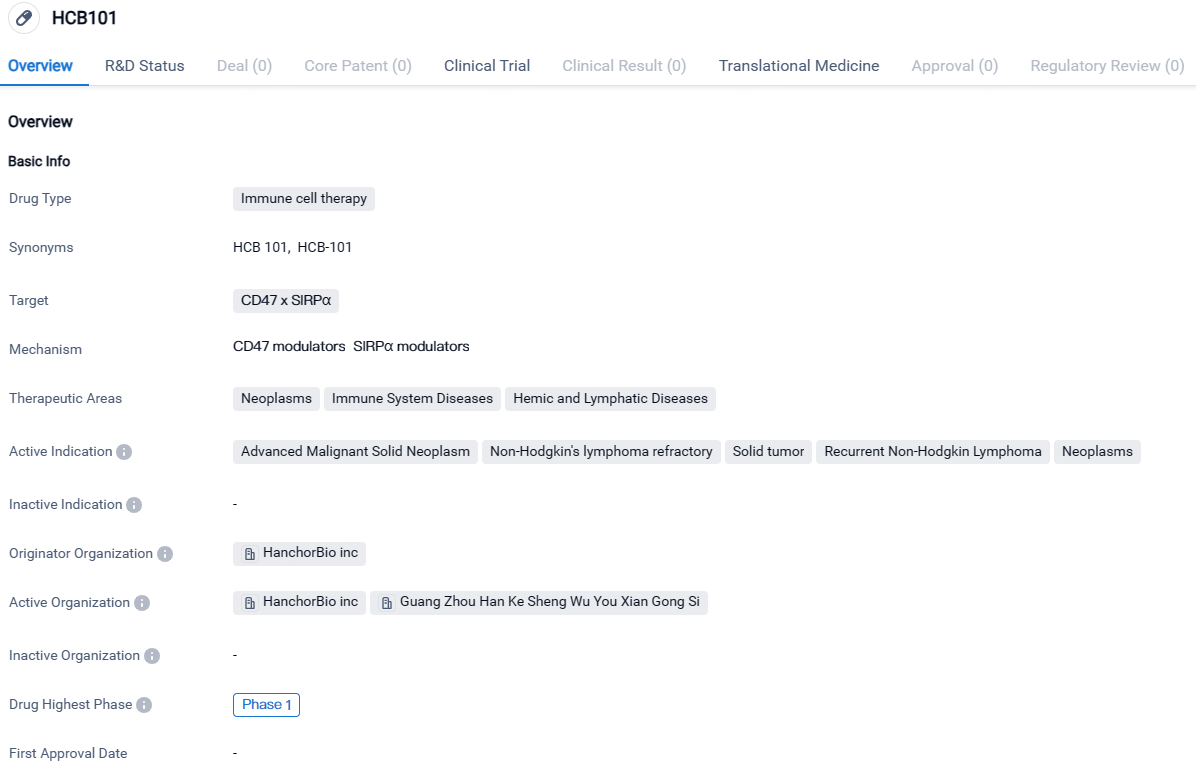
HCB101 targets CD47 x SIRPα and is intended for the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. While it is currently in Phase 1 of development globally, it has received IND approval in China, indicating progress in its development in that market.