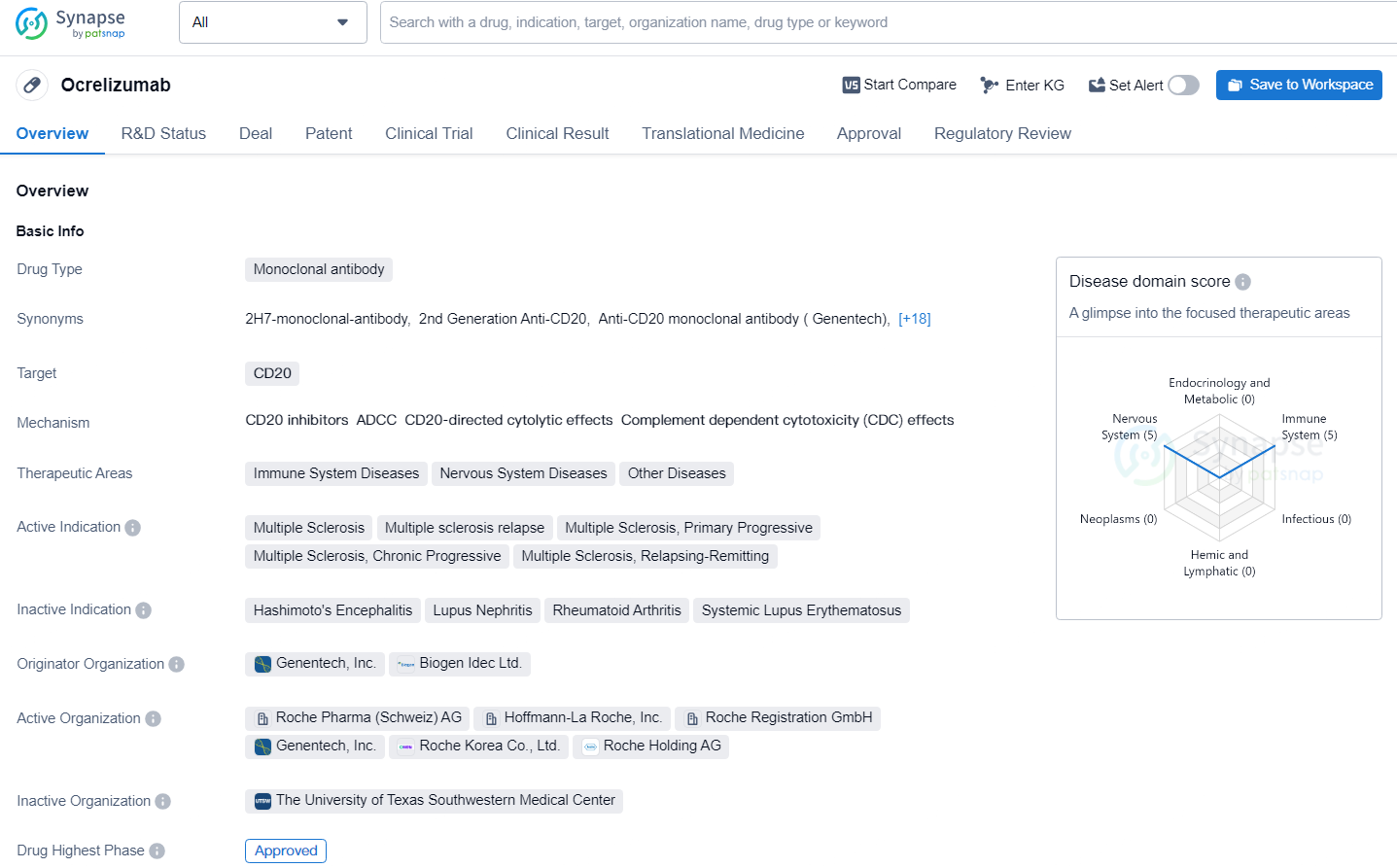
EU Commission Approves Roche's OCREVUS: First Biannual Injection for Multiple Sclerosis
Roche has revealed that the European Commission has endorsed the marketing of OCREVUS® (ocrelizumab) in subcutaneous form for addressing both relapsing multiple sclerosis and primary progressive multiple sclerosis. This new subcutaneous version, OCREVUS SC, offers a quick 10-minute injection, adhering to the same biannual regimen as the earlier sanctioned intravenous infusion. Globally, over 350,000 individuals with multiple sclerosis have undergone treatment with OCREVUS IV.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"OCREVUS has revolutionized multiple sclerosis treatment as the first anti-CD20 therapy approved for this condition. Currently, individuals in the EU with multiple sclerosis can have their medication administered in just 10 minutes twice yearly without needing an intravenous facility," said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. "This advancement simplifies treatment access for patients and saves time for healthcare providers."
The approval is founded on crucial data from the Phase III OCARINA II trial, which demonstrated equivalent levels of OCREVUS in the bloodstream when given subcutaneously. The trial also showed a safety and efficacy profile similar to the intravenous form in patients with RMS and PPMS. OCREVUS SC was well tolerated, with no new safety issues identified. Over 92% of participants in the study reported being satisfied or very satisfied with the subcutaneous administration of OCREVUS.
OCREVUS SC was developed to offer an alternative biannual treatment option besides IV, allowing the administration method to be tailored to the needs of patients and healthcare professionals. The SC injection is designed to be administered by healthcare providers, either in a clinic or in alternative settings.
Roche remains dedicated to advancing innovative clinical research programs to enhance the scientific understanding of multiple sclerosis, further reduce disability progression in RMS and PPMS, and improve treatment experiences for those affected by multiple sclerosis.
OCREVUS is a humanized monoclonal antibody targeted at CD20-positive B cells, a specific type of immune cell believed to play a significant role in myelin and axonal damage. This damage to nerve cells can result in disability in individuals with multiple sclerosis. According to preclinical studies, OCREVUS binds to CD20 cell surface proteins on certain B cells but not on stem cells or plasma cells, indicating that essential immune system functions might be preserved.
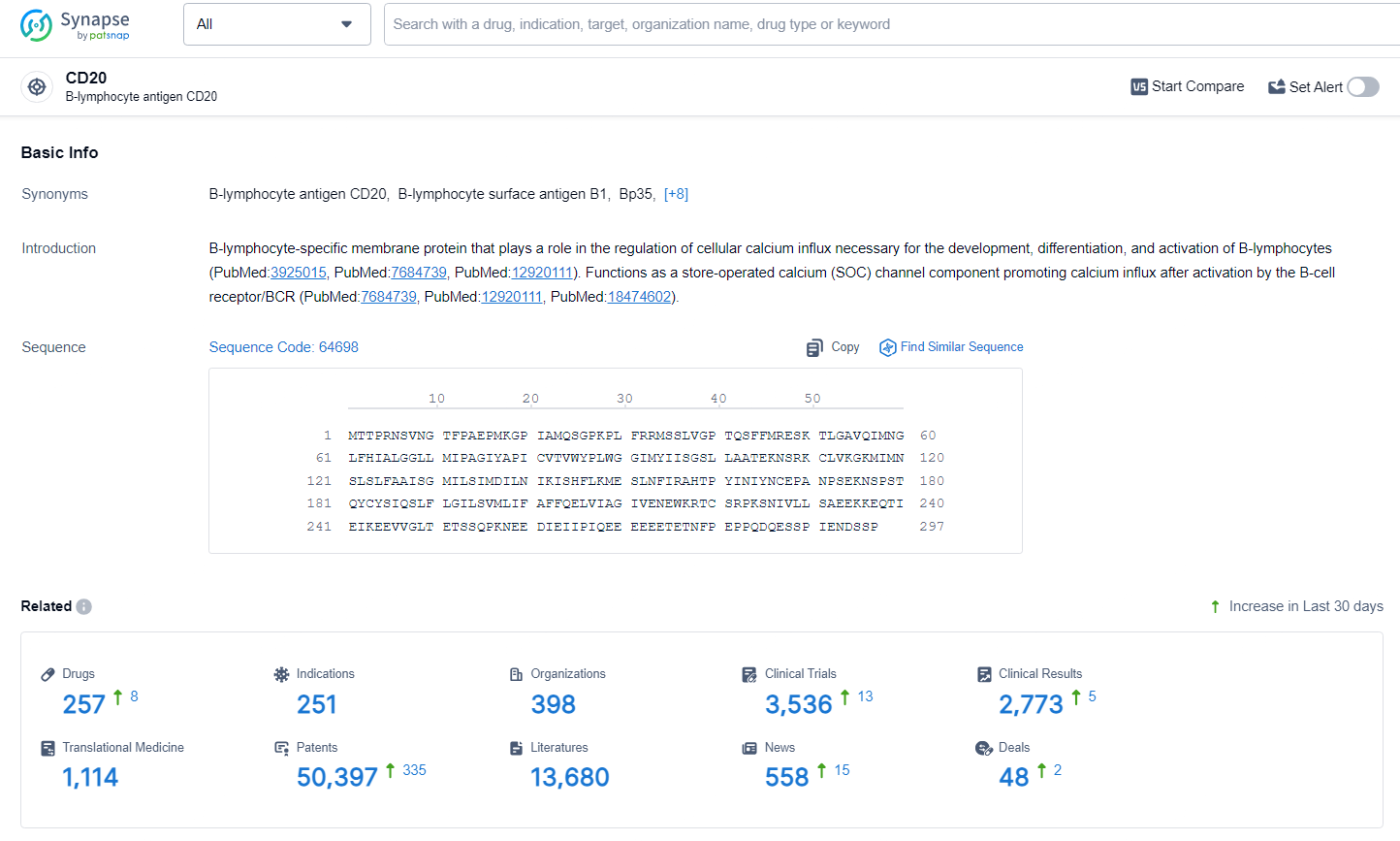
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 28, 2024, there are 257 investigational drugs for the CD20 target, including 251 indications, 398 R&D institutions involved, with related clinical trials reaching 3536, and as many as 50397 patents.
Ocrelizumab is a monoclonal antibody drug that has been approved for the treatment of multiple sclerosis and has shown promise in addressing unmet medical needs in this therapeutic area. Its approval in the United States and ongoing review in China, as well as its Fast Track designation, underscore the potential of Ocrelizumab as a valuable treatment option for patients with multiple sclerosis and related conditions.