Is Isatuximab approved by the FDA?
Yes, Isatuximab is FDA approved. The U.S. Food and Drug Administration (FDA) approved Isatuximab, under the brand name Sarclisa, on March 2, 2020. This medication is classified as a CD38 monoclonal antibody and is specifically used to treat multiple myeloma in adults.
What is Isatuximab?
Isatuximab is a prescription medication designed to treat multiple myeloma, a type of blood cancer, in adults. It is typically administered in combination with other cancer medications, such as carfilzomib or pomalidomide, along with the steroid dexamethasone. Isatuximab is used after other cancer treatments have failed or stopped working.
How Does Isatuximab Work?
Isatuximab targets and binds to a specific protein called CD38, which is found on the surface of multiple myeloma cells. By binding to CD38, Isatuximab helps the immune system identify and destroy these cancer cells. This mechanism helps in managing and reducing the progression of multiple myeloma.
Dosage and Administration
Isatuximab is administered as an intravenous infusion. The typical dosage schedule is as follows:
- Cycle 1: 10 mg/kg IV on Days 1, 8, 15, and 22 (weekly).
- Cycle 2 and Beyond: 10 mg/kg IV on Days 1 and 15 (every 2 weeks).
Each treatment cycle consists of a 28-day period, and therapy is continued until disease progression or unacceptable toxicity occurs.
Usage Guidelines
- Premedication: Patients are given premedications like dexamethasone, acetaminophen, H2 antagonists, and diphenhydramine 15 to 60 minutes before the infusion to prevent serious side effects or allergic reactions.
- Monitoring: Patients are closely monitored for at least 30 minutes after the first two infusions to check for any allergic reactions.
Warnings and Precautions
- Allergies: Do not use Isatuximab if you are allergic to it.
- Pregnancy: Isatuximab can harm an unborn baby. Effective birth control should be used during treatment and for at least 5 months after the last dose.
- Breastfeeding: Breastfeeding is not recommended while using Isatuximab.
- Other Cancers: Using Isatuximab may increase the risk of developing other cancers.
Side Effects
Common side effects of Isatuximab include:
- Low blood cell counts
- Pneumonia
- Diarrhea
- Cold symptoms (stuffy nose, sneezing, sore throat)
Serious side effects that require immediate medical attention include:
- Allergic reactions (hives, difficulty breathing, swelling of the face, lips, tongue, or throat)
- Low white or red blood cell counts, leading to symptoms like fever, mouth sores, skin sores, sore throat, and anemia
- Symptoms of pneumonia, such as a cough with mucus, chest pain, and shortness of breath
Drug Interactions
Isatuximab can interact with other medications, including prescription and over-the-counter drugs, vitamins, and herbal products. It is crucial to inform your healthcare provider about all medications you are taking.
Conclusion
Isatuximab (Sarclisa) is an FDA-approved medication for the treatment of multiple myeloma in adults. Approved on March 2, 2020, it is used in combination with other cancer therapies after previous treatments have failed. Patients should follow all prescribed guidelines and consult their healthcare provider for personalized advice and monitoring during treatment.
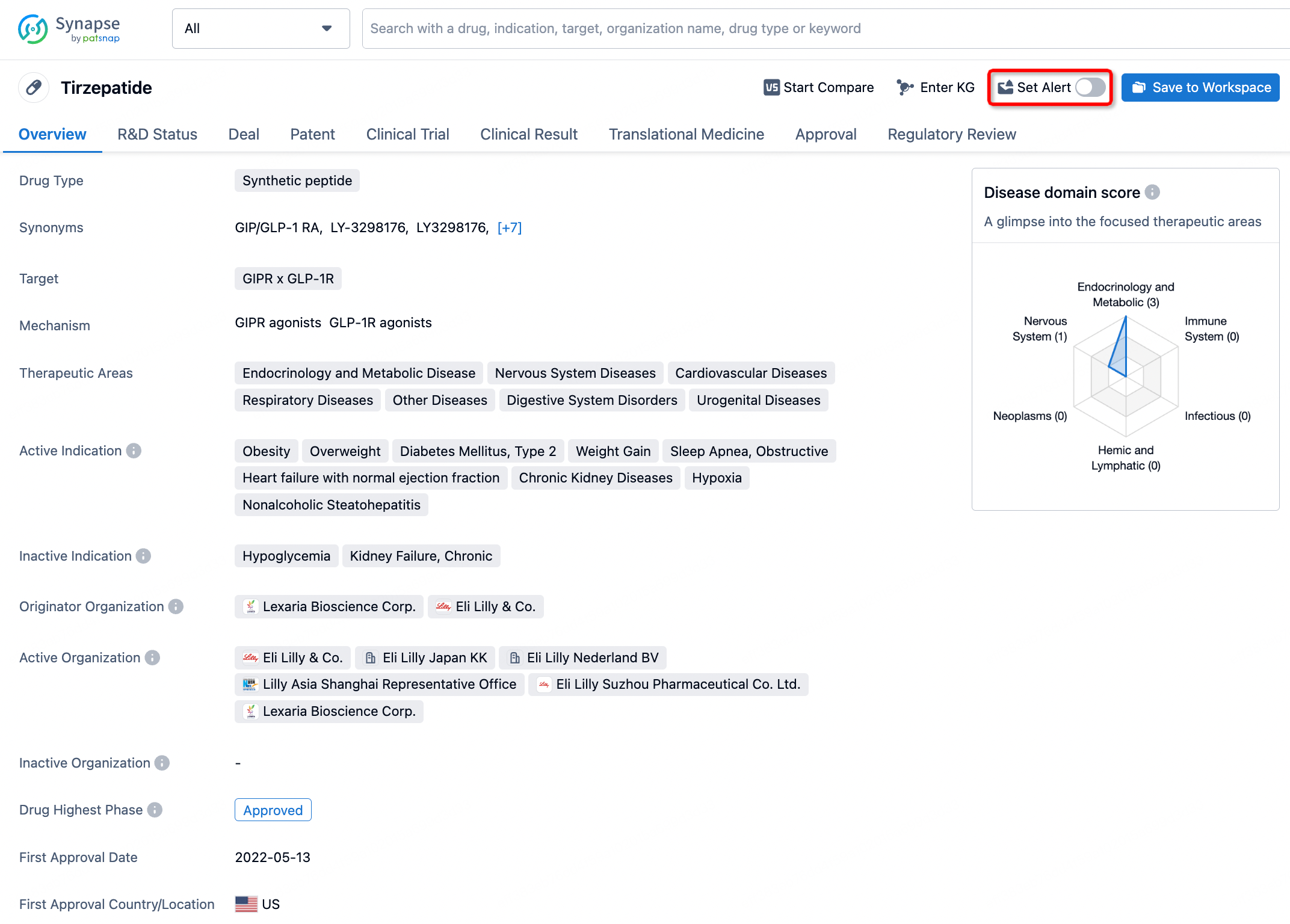
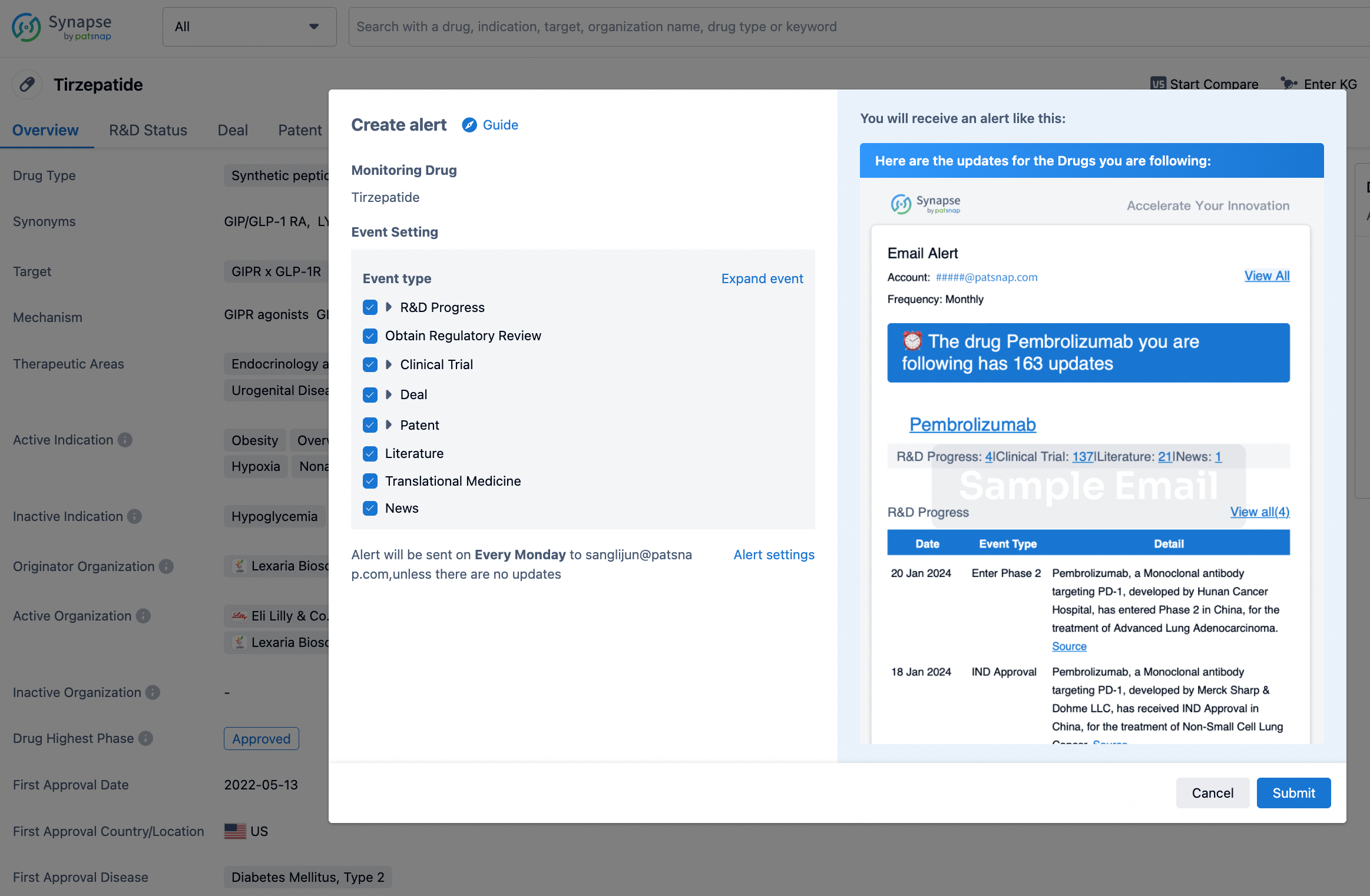
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!