Exploring Casdatifan: A Promising Small Molecule in the Fight Against Cancer and its Emerging Patent Landscape
Casdatifan is a small molecule drug designed to target HIF-2α, and it is being developed for the treatment of neoplasms, urogenital diseases, and digestive system disorders. The drug is specifically indicated for renal cell carcinoma and liver cancer. Casdatifan was originated by Arcus Biosciences, Inc., a biopharmaceutical company focused on the discovery and development of innovative cancer immunotherapies. As of the latest available information, the drug has reached Phase 1 in its development, indicating that it has successfully gone through preclinical studies and is now being tested for safety and efficacy in humans. This phase represents an early stage in the drug development process, where the focus is on determining the optimal dose and evaluating potential side effects.
Below, we will use the drug Casdatifan as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
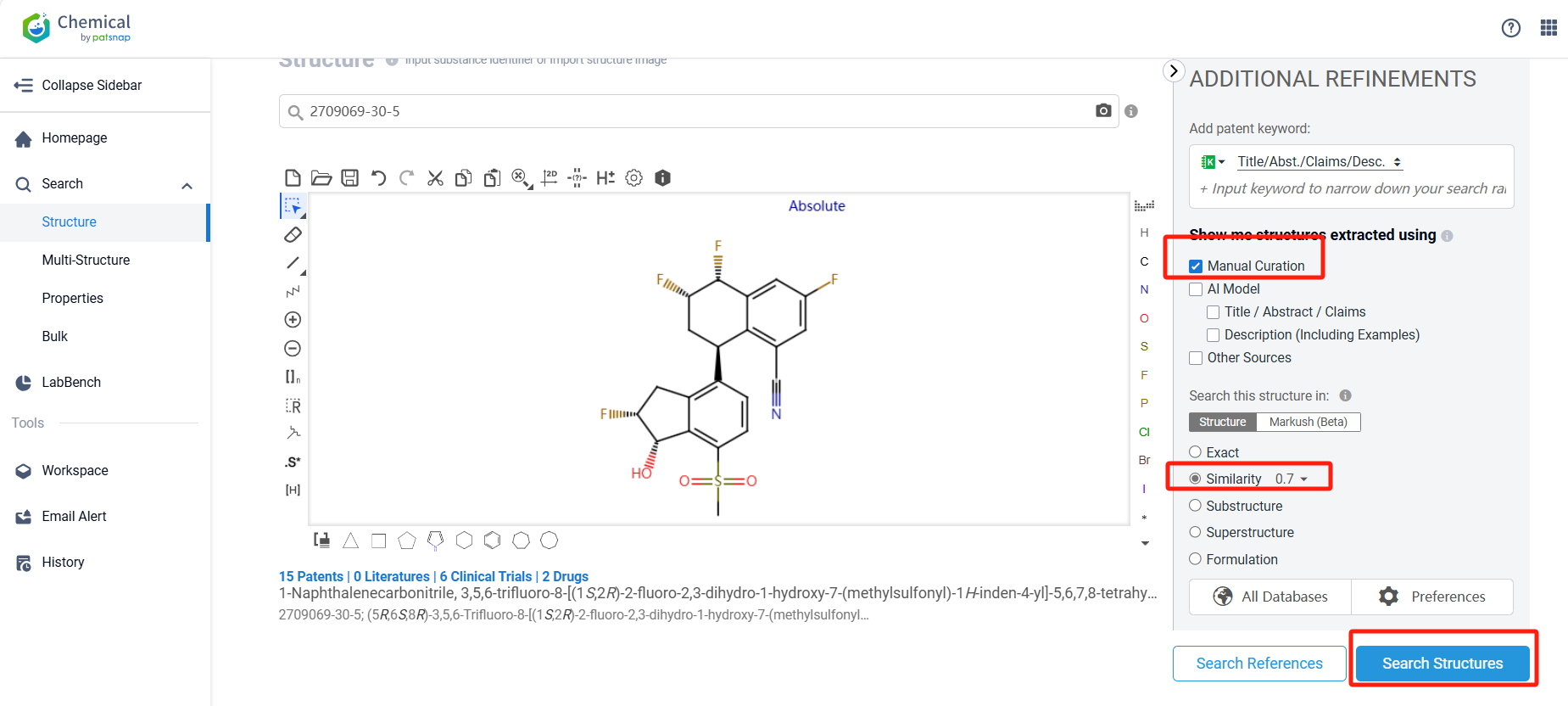
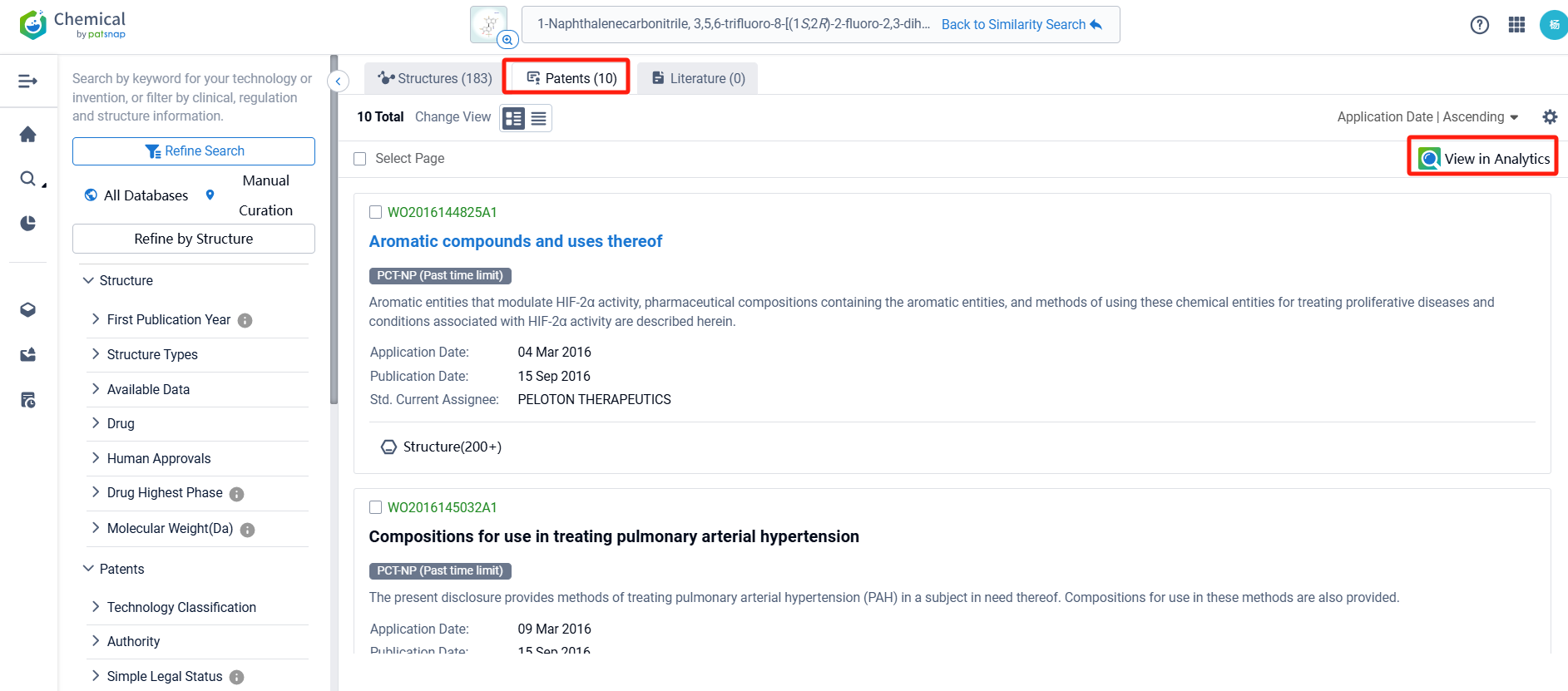
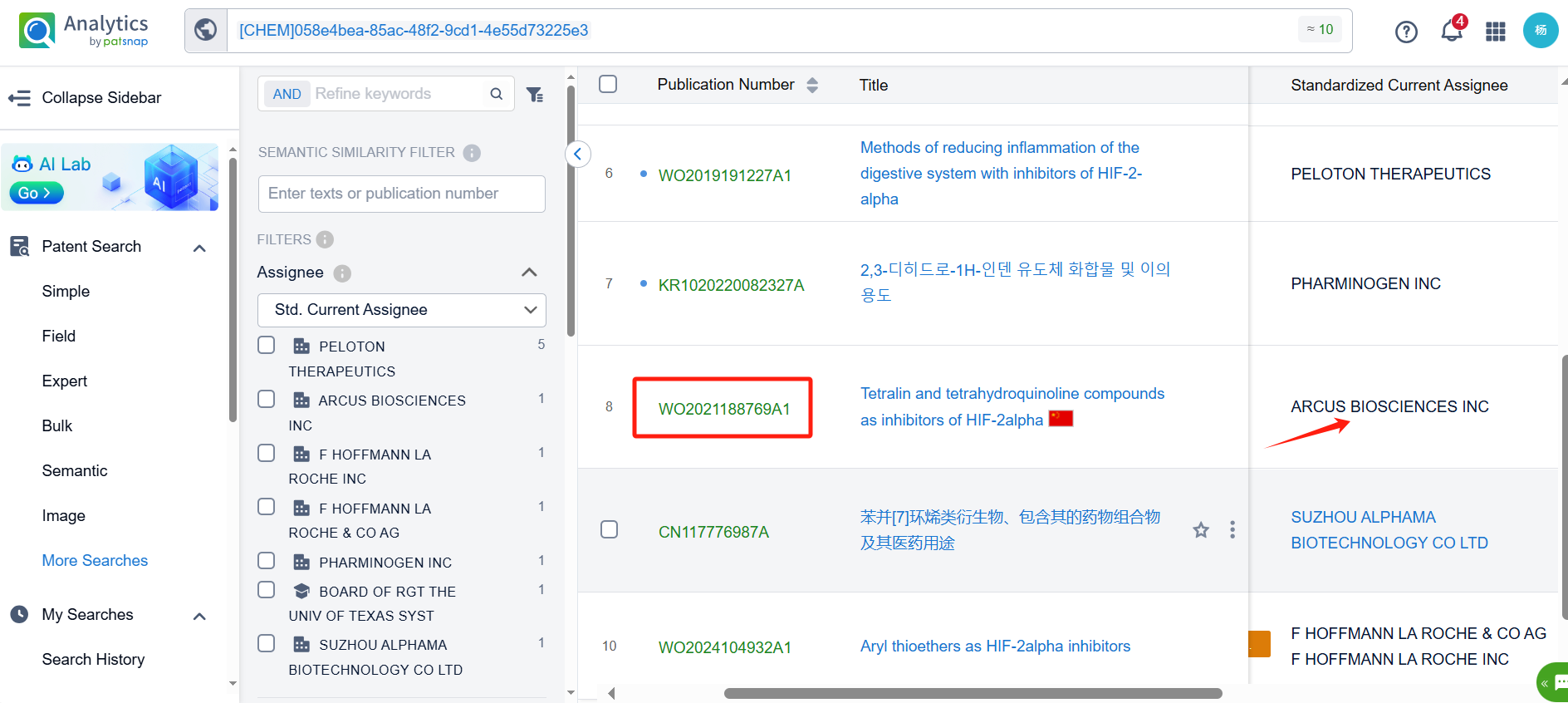
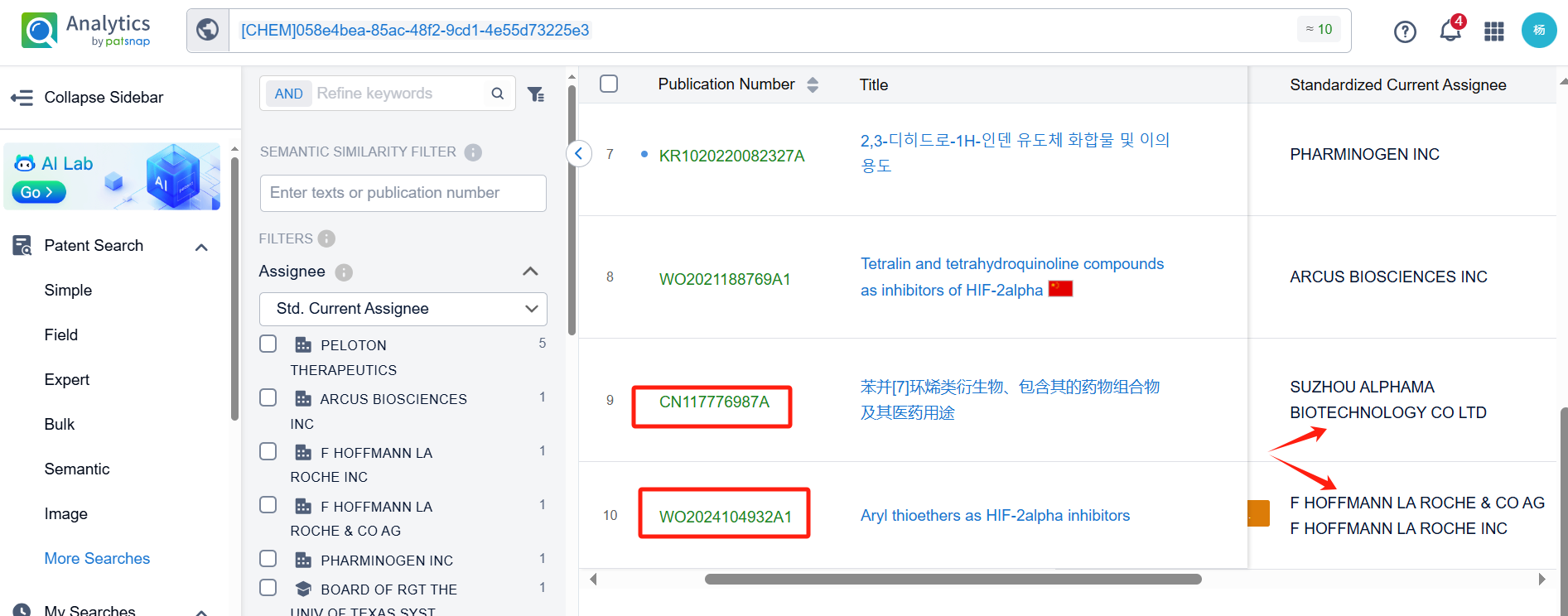
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Casdatifan (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, using a similarity search (setting the Tanimoto coefficient to 0.7), check the box for manual curation, click on search structures, and you can find 10 patents. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
As a Business Development expert in the pharmaceutical industry, it is crucial to closely monitor the progress of drugs like Casdatifan, particularly at the early stages of development. Understanding the specific therapeutic targets, active indications, and the developmental phase of a drug allows for informed assessments of its potential market impact and strategic partnerships. Additionally, keeping abreast of advancements in the biomedicine field, such as the development of small molecule drugs targeting specific pathways, is essential for identifying opportunities for collaboration, licensing, or investment in promising drug candidates. The information provided about Casdatifan serves as a valuable point of reference for evaluating its potential business prospects within the pharmaceutical industry.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.