Sutro Biopharma Initiates REFRαME-P1 Trial for Pediatric CBF/GLIS AML Registration of Luvelta
Sutro Biopharma, Inc. (NASDAQ: STRO), a clinical-stage oncology firm specializing in innovative site-specific antibody drug conjugates (ADCs), has announced the initiation of the REFRαME-P1 trial. This registration-focused study is evaluating luveltamab tazevibulin (luvelta) for pediatric patients diagnosed with CBFA2T3::GLIS2 (CBF/GLIS; RAM phenotype) acute myeloid leukemia (AML) and is currently open for participant enrollment.
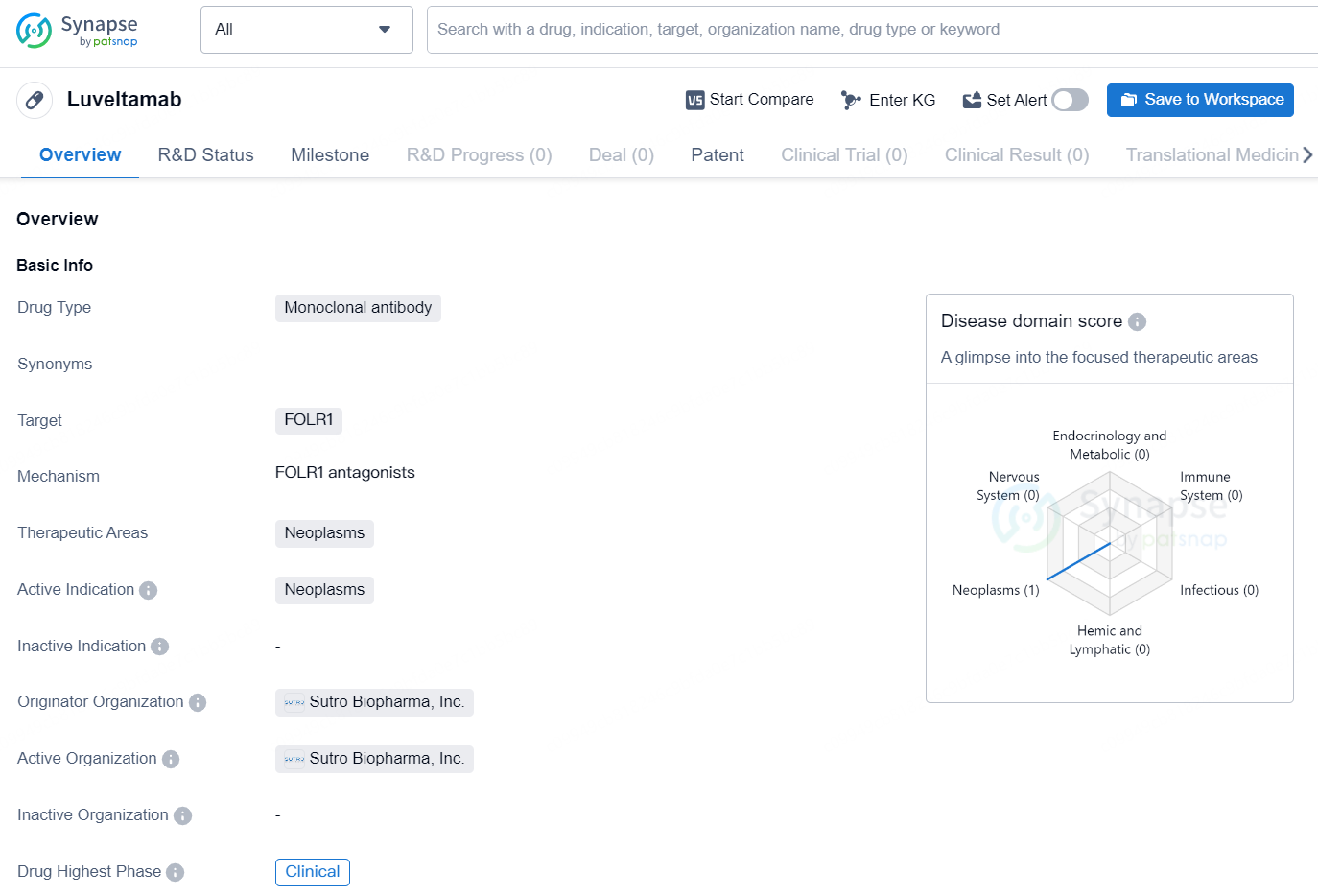
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“We are thrilled to share the launch of our second significant trial, a clinical study aimed at obtaining registration for luvelta in infants and toddlers suffering from a rare and aggressive leukemia,” stated Anne Borgman, M.D., Chief Medical Officer at Sutro. “We are eager to provide this promising targeted treatment to pediatric patients who currently have few effective options available.”
“Commencing this trial marks a crucial advancement in the clinical development journey of luvelta, offering solutions for various cancers expressing Folate Receptor-α (FRα) beyond just ovarian cancer,” remarked Soheil Meshinchi, M.D., Ph.D. “As someone focused on AML biology, I feel privileged to have contributed towards making this therapy accessibly through a compassionate use program led by Sutro, which has shown optimistic outcomes in this challenging disease.”
In December 2023, Dr. Meshinchi revealed findings on the anti-leukemic effects of luvelta from the compassionate use in 25 pediatric patients experiencing relapsed/refractory CBFA2T3-GLIS2 (CBF/GLIS) acute myeloid leukemia (AML) at the American Society of Hematology Annual Meeting and Exposition. The results indicated that luvelta treatment led to significant clinical responses, with a complete remission rate of 42% in patients with ≥5% blasts, and an extension of overall survival, allowing some patients to undergo hematopoietic stem cell transplants, a potentially life-saving intervention.
The CBF/GLIS subtype of AML is an uncommon and highly aggressive leukemia prevalent only in infants and young children, typically diagnosed around 18 months. There are currently no specific therapies approved for this type of leukemia, and it shows significant resistance to standard chemotherapy, with an induction failure rate exceeding 80%. Due to insufficient treatment options, the prognosis for children with this diagnosis is grim, with a mere two-year survival rate. Recent research has identified that FOLR1, which encodes FRα, is inactive during normal hematopoiesis, yet is specifically activated by the CBF/GLIS gene fusion.
REFRaME-P1 is a study enabling registration that assesses the safety and effectiveness of luvelta in infants and children under 12 years old diagnosed with CBF/GLIS AML. This global trial aims to have most of its sites operational by the year's end.
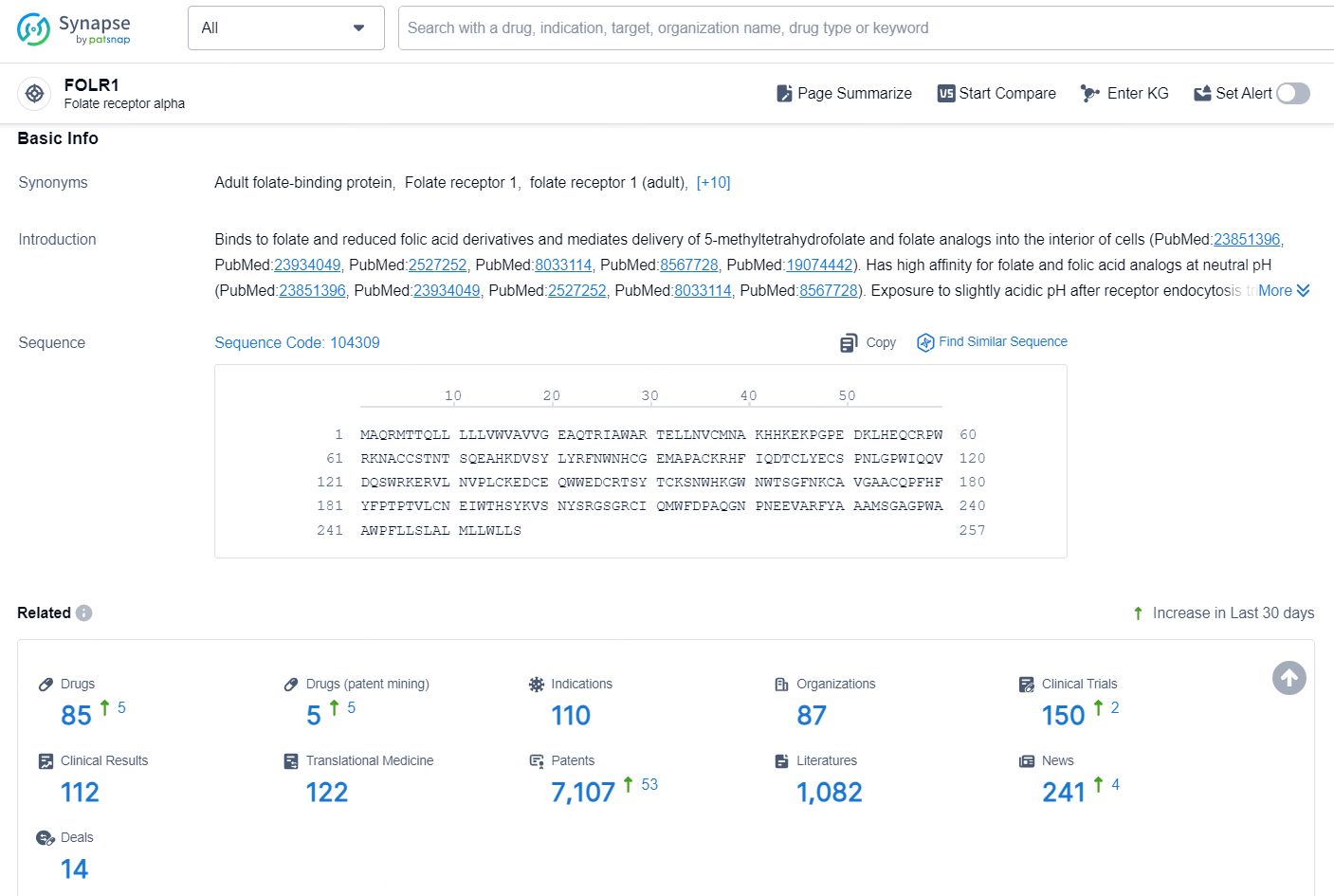
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 5, 2024, there are 85 investigational drugs for the FOLR1 target, including 110 indications, 87 R&D institutions involved, with related clinical trial reaching 150, and as many as 7107 patents.
Luveltamab is a monoclonal antibody drug developed by Sutro Biopharma, Inc. that targets FOLR1 and is intended for the treatment of neoplasms, or tumors. As a monoclonal antibody, Luveltamab is designed to specifically target and bind to the FOLR1 protein, which is often overexpressed in certain types of cancer cells. This targeted approach aims to inhibit the growth and survival of cancer cells while minimizing harm to healthy cells.