Exploring Cefadroxil's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Cefadroxil's R&D Progress
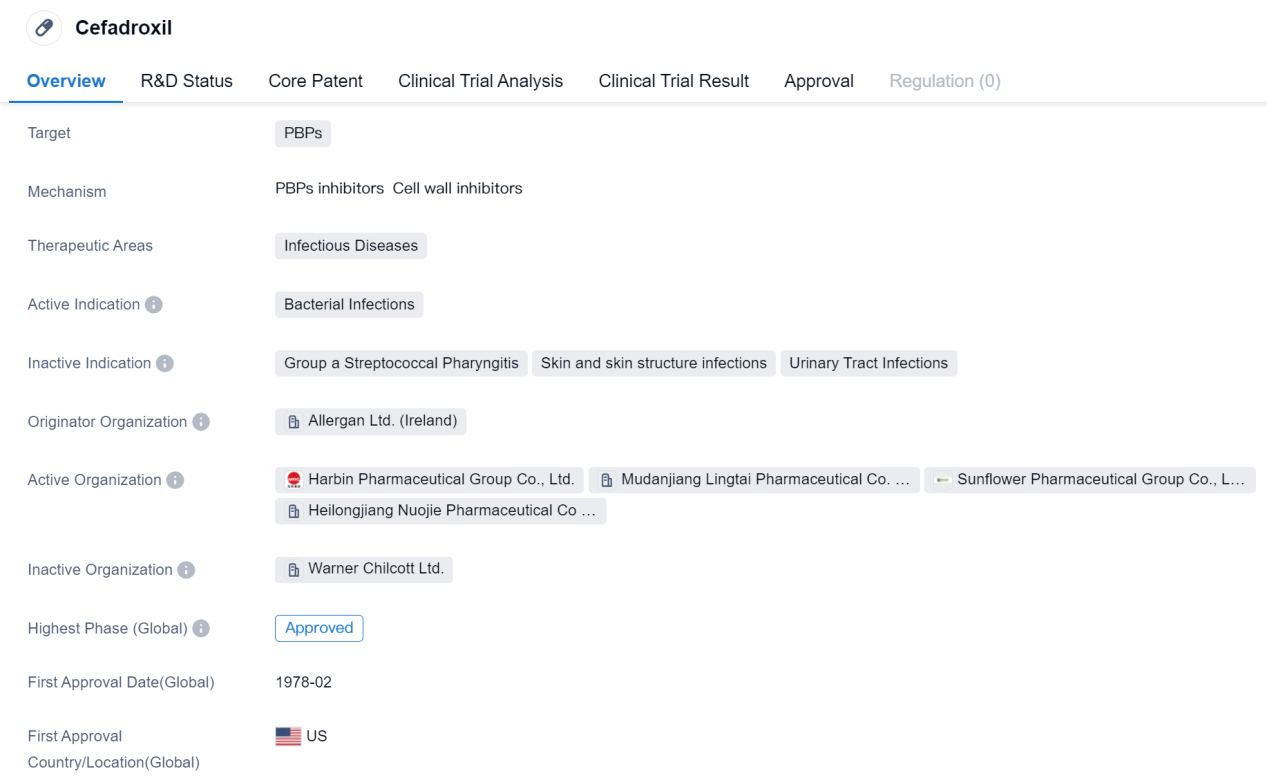
Cefadroxil is a small molecule drug that falls under the category of biomedicine. It primarily targets PBPs (penicillin-binding proteins) and is used in the treatment of infectious diseases, specifically bacterial infections. The drug was first approved in the United States in February 1978 and has since gained approval in various other countries.
The originator organization of Cefadroxil is Allergan Ltd., based in Ireland. Allergan Ltd. is responsible for the development and initial marketing of the drug. Cefadroxil has reached the highest phase of approval globally, indicating its widespread acceptance and recognition in the pharmaceutical industry.
As a small molecule drug, Cefadroxil is designed to interact with specific targets, in this case, PBPs. PBPs are enzymes found in bacterial cell walls that play a crucial role in cell wall synthesis. By targeting PBPs, Cefadroxil disrupts the bacterial cell wall formation, leading to the inhibition of bacterial growth and ultimately the eradication of the infection.
The therapeutic area of Cefadroxil is focused on infectious diseases, particularly bacterial infections. This drug is commonly prescribed for various types of bacterial infections, including respiratory tract infections, skin and soft tissue infections, urinary tract infections, and other common bacterial infections.
Cefadroxil's approval in multiple countries, highlights its global recognition and effectiveness in treating bacterial infections. Its long history of approval since 1978 further demonstrates its established position in the pharmaceutical market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Cefadroxil: PBPs inhibitors and Cell wall inhibitors
From a biomedical perspective, PBPs inhibitors refer to a class of drugs that target penicillin-binding proteins (PBPs). PBPs are enzymes found in the cell wall of bacteria and are involved in the synthesis of peptidoglycan, a crucial component of the bacterial cell wall. By inhibiting PBPs, these drugs prevent the proper formation of the bacterial cell wall, leading to cell lysis and ultimately bacterial death. PBPs inhibitors are commonly used antibiotics in the treatment of bacterial infections.
Cell wall inhibitors, on the other hand, are a broader category of drugs that target various components involved in the formation of the bacterial cell wall. In addition to PBPs inhibitors, other types of cell wall inhibitors include drugs that inhibit enzymes involved in peptidoglycan synthesis, such as glycopeptide antibiotics, as well as drugs that disrupt the integrity of the cell wall, such as beta-lactam antibiotics. These drugs work by interfering with the structural integrity of the bacterial cell wall, making it more susceptible to damage and leading to bacterial death. Cell wall inhibitors are an important class of antibiotics used in the treatment of bacterial infections.
Drug Target R&D Trends for Cefadroxil
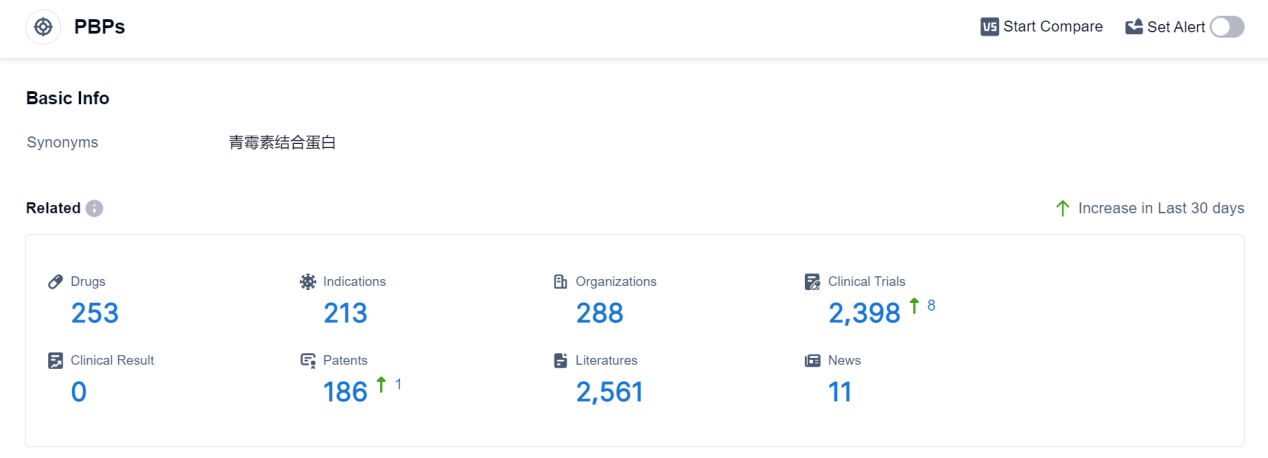
PBPs, or Penicillin-Binding Proteins, play a crucial role in the human body. These proteins are found in the cell walls of bacteria and are responsible for the synthesis and maintenance of the peptidoglycan layer, a vital component of bacterial cell walls. PBPs act as targets for antibiotics, particularly beta-lactam antibiotics like penicillin, by binding to them and inhibiting the enzymes that cross-link the peptidoglycan strands. This disruption weakens the bacterial cell wall, leading to cell lysis and ultimately bacterial death. Understanding the role of PBPs is essential in developing effective antibiotics and combating bacterial infections.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 253 PBPs drugs worldwide, from 288 organizations, covering 213 indications, and conducting 2398 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Cefadroxil is a small molecule drug developed by Allergan Ltd. that targets PBPs to treat bacterial infections. It has gained approval in various countries, and has been used effectively since its first approval in 1978. With its proven efficacy and widespread acceptance, Cefadroxil continues to be an important drug in the field of infectious diseases.