Observations on the Use of CB1 Antagonists in Disease Treatment
The Cannabinoid receptor 1 (CB1), as one of the most classic receptors of the Endocannabinoid system (ECS), is a G protein-coupled membrane receptor. The CB1 gene is located on chromosome 6q14-q15, containing 4 exons and 3 introns. The encoded CB1 protein is composed of 473 amino acids, with the 117 N-terminal amino acids forming the extracellular region, and the 401-473 C-terminal amino acids forming the intracellular region, with seven transmembrane domains in between. The extracellular region also contains three hydrophilic structural domains formed by the seven transmembrane domains: e1, e2, and e3, among which e2 is considered the functional domain that binds to cannabinoids. When cannabinoids act on CB1, they activate multiple intracellular signal transduction pathways, exerting various physiological and pathological functions.
Upon stimulation or binding by cannabinoids and their derivatives, CB1 activates intracellular signals, mediating the ECS's extensive and complex biological functions. For example, CB1 plays an essential regulatory role in memory, cognition, motion, and emotion; CB1 regulates the levels of the second messenger AMP in the adenylyl cyclase system, thus influencing cell function, substance metabolism, immune function, and gene expression. CB1 also adjusts the calcium ion level through the intracellular calmodulin system. Calcium ions play a critical role in cellular function, including affecting signal transduction, tumor development, and autophagy. In recent years, these discoveries have injected new research value into CB1, a classic protein that has been discovered for over 30 years!
CB1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 106 CB1 drugs worldwide, from 105 organizations, covering 110 indications, and conducting 412 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.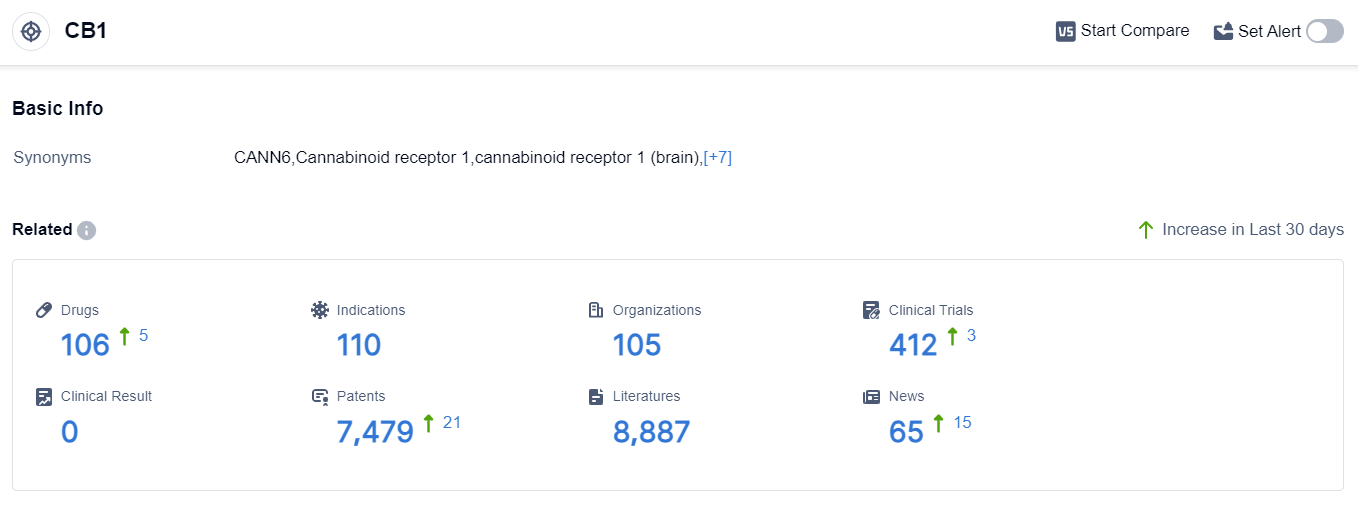
Based on the analysis of the provided data, the current competitive landscape of target CB1 in the pharmaceutical industry is characterized by the involvement of multiple companies at different stages of development. Sanofi is the leading company with drugs approved and in Phase 3.
The indications for drugs under target CB1 are diverse, including obesity, neuralgia, cancer pain, and various other conditions. Small molecule drugs are the most rapidly progressing drug type, followed by monoclonal antibodies, diagnostic radiopharmaceuticals, unknown drugs, and chemical drugs.
The development of target CB1 is happening globally, with significant progress in countries such as the European Union, Canada, Germany, Mexico, and others. The future development of target CB1 will depend on the success of ongoing clinical trials and the ability of companies to bring innovative drugs to market.
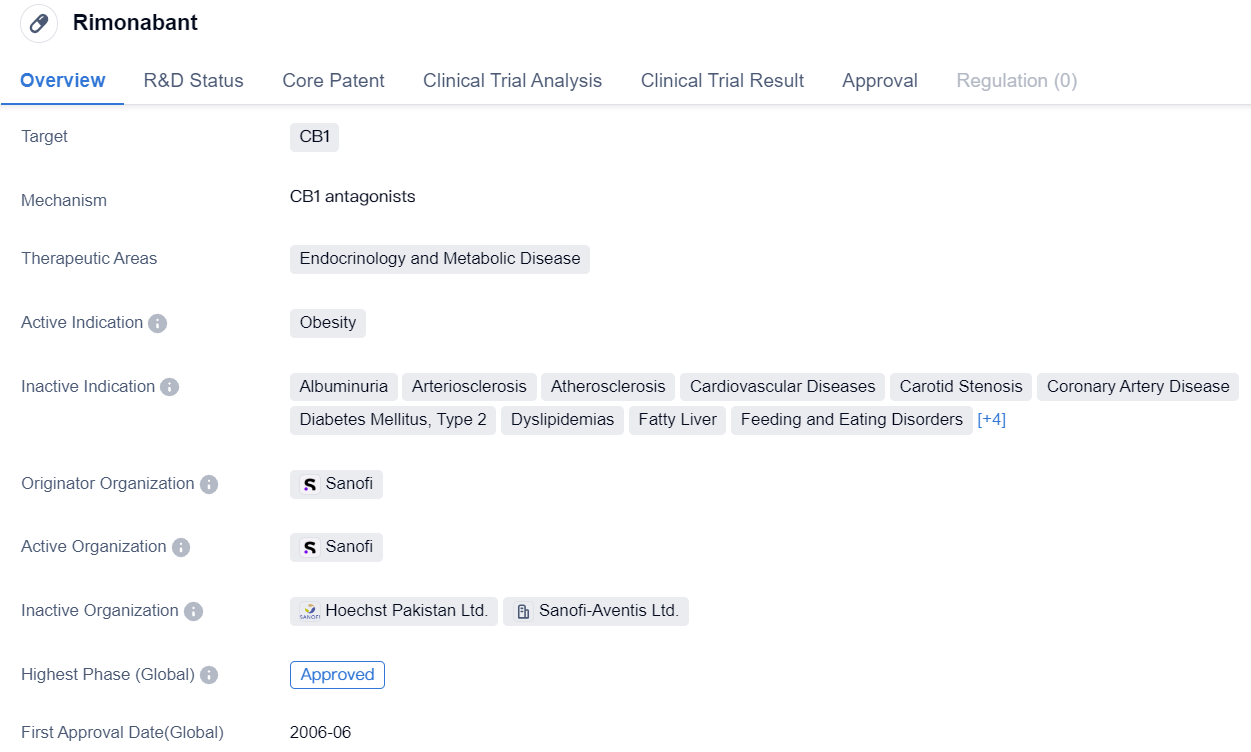
Key Drug: Rimonabant
Rimonabant is a small molecule drug that targets the CB1 receptor and is primarily used in the therapeutic areas of endocrinology and metabolic disease. Its active indication is obesity. The drug was developed by Sanofi, a pharmaceutical company. Rimonabant has reached the highest phase of approval globally, indicating that it has successfully completed clinical trials and has been deemed safe and effective for use in patients. The drug received its first approval in the European Union in June 2006.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Rimonabant's mechanism of action involves targeting the CB1 receptor, which is primarily found in the central nervous system. By blocking this receptor, the drug is believed to reduce appetite and food intake, making it a potential treatment option for obesity. However, it is important to note that the drug's development in China has been discontinued, suggesting that it may not have met the necessary requirements or faced challenges in the Chinese market.
The approval of Rimonabant in the European Union in 2006 marked an important milestone for the drug and its potential impact on the treatment of obesity. Obesity is a significant global health issue, with increasing prevalence and associated complications such as diabetes and cardiovascular diseases. Therefore, the approval of a drug specifically targeting obesity is a significant development in the field of endocrinology and metabolic disease.
Sanofi, as the originator organization of Rimonabant, played a crucial role in the drug's development and successful completion of clinical trials. The highest phase of approval achieved globally indicates that the drug has undergone rigorous testing and has met the necessary safety and efficacy standards.
In summary, Rimonabant is a small molecule drug that targets the CB1 receptor and is used in the therapeutic areas of endocrinology and metabolic disease, specifically for the treatment of obesity. Developed by Sanofi, the drug received its first approval in the European Union in 2006 and has reached the highest phase of approval globally. While its development in China has been discontinued, the drug's approval in the European Union highlights its potential impact on addressing the global issue of obesity.
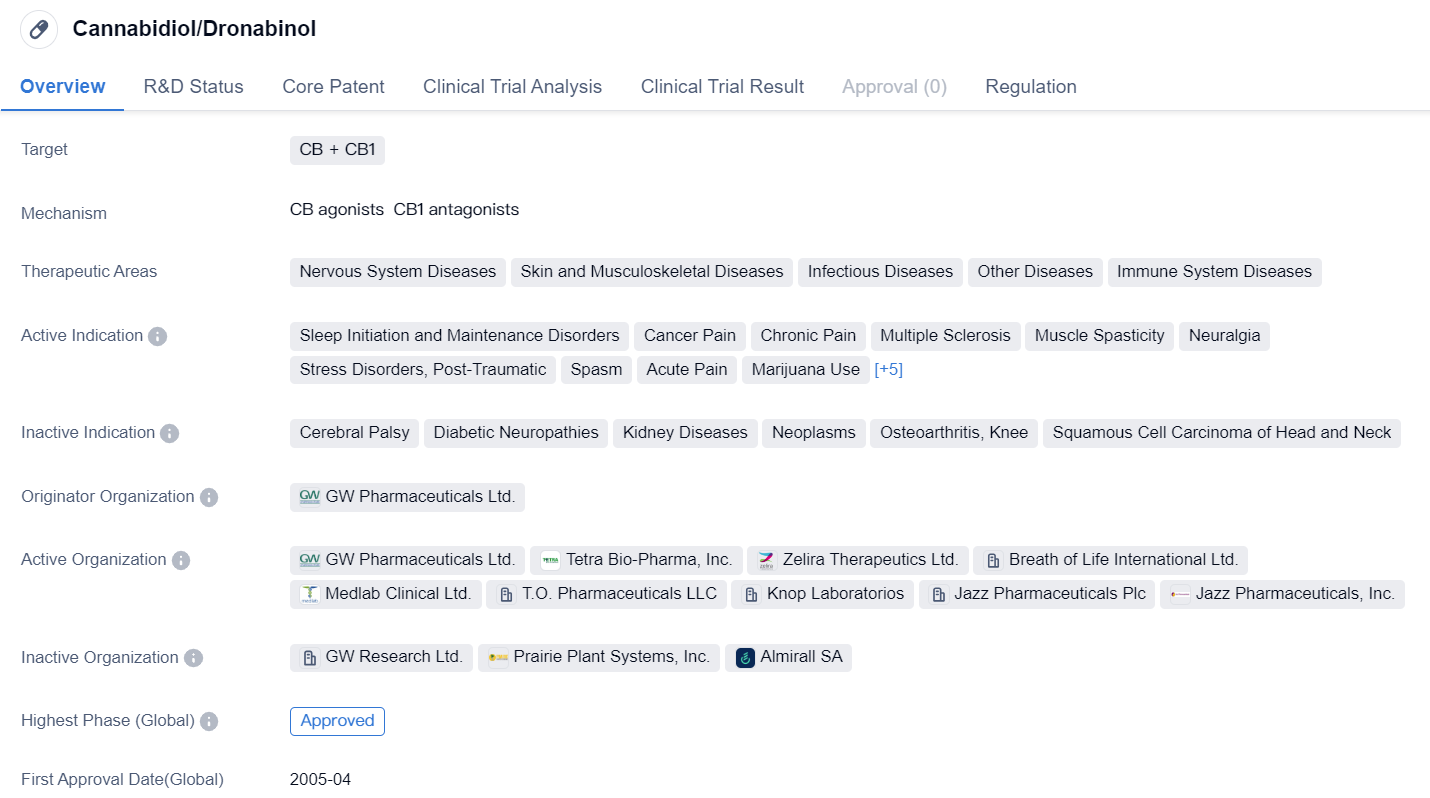
Cannabidiol/Dronabinol
Cannabidiol (CBD) /Dronabinol are small molecule drugs that target the CB and CB1 receptors in the body. These drugs have shown potential in treating various therapeutic areas including Nervous System Diseases, Skin and Musculoskeletal Diseases, Infectious Diseases, Other Diseases, and Immune System Diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The active indications for CBD/Dronabinol include Sleep Initiation and Maintenance Disorders, Cancer Pain, Chronic Pain, Multiple Sclerosis, Muscle Spasticity, Neuralgia, Stress Disorders (Post-Traumatic), Spasm, Acute Pain, Marijuana Use, Hidradenitis Suppurativa, Low Back Pain, Sciatica, Neuralgia (Postherpetic), and Fibromyalgia.
GW Pharmaceuticals Ltd. is the originator organization of these drugs. CBD received its first approval in Canada in April 2005, making it the first country/location to approve the drug globally. However, the highest phase of approval for CBD/Dronabinol in China is still pending.
It is worth noting that CBD/Dronabinol are regulated as orphan drugs. Orphan drug status is granted to drugs that are intended to treat rare diseases or conditions, providing incentives to pharmaceutical companies to develop and market these drugs.
In summary, CBD/Dronabinol are small molecule drugs that target CB and CB1 receptors. They have shown potential in treating various therapeutic areas, including nervous system diseases, skin and musculoskeletal diseases, infectious diseases, and immune system diseases. CBD received its first approval in Canada in 2005 and is regulated as an orphan drug. The highest phase of approval for these drugs in China is still pending.