Exploring lusutrombopag's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Lusutrombopag's R&D Progress
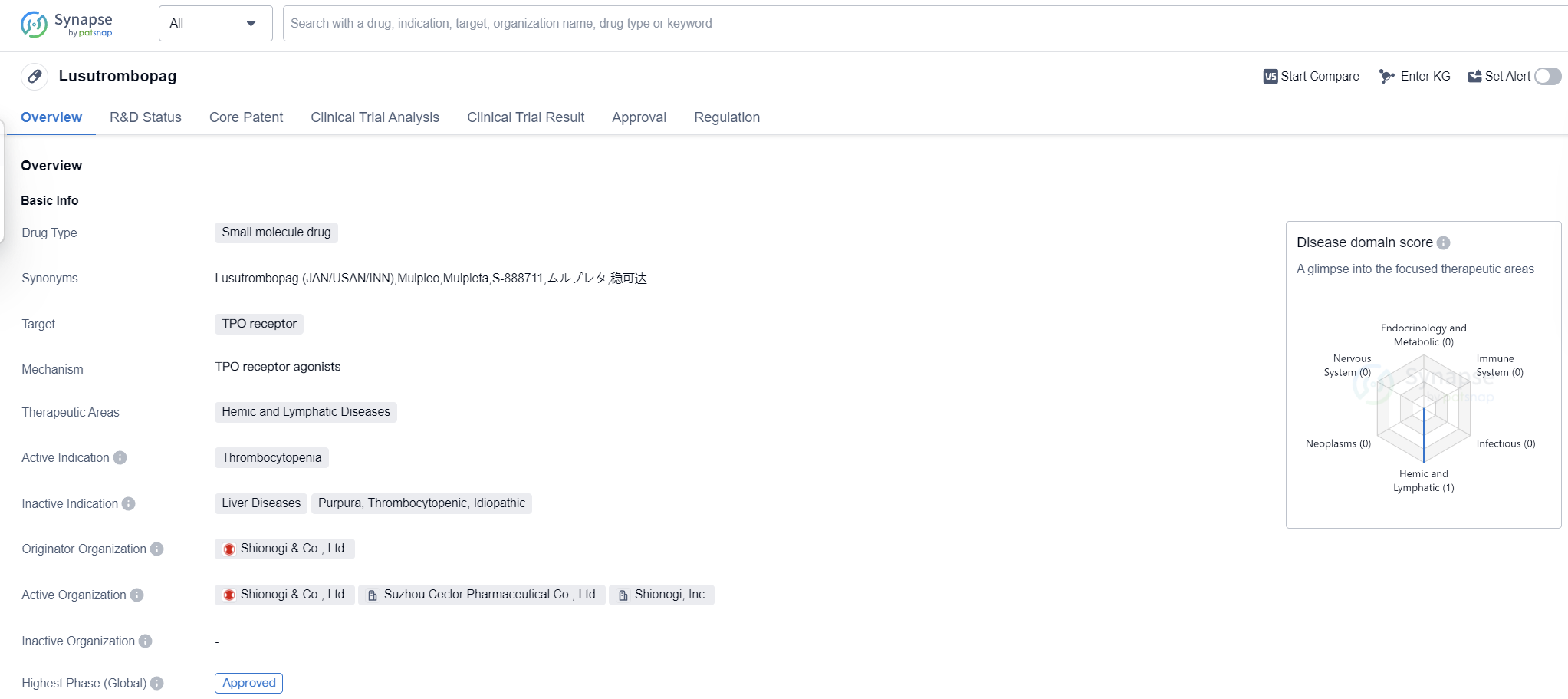
Lusutrombopag is a small molecule drug that targets the TPO receptor and is used in the treatment of thrombocytopenia, a condition characterized by low platelet count. It falls under the therapeutic area of hemic and lymphatic diseases. The drug was developed by Shionogi & Co., Ltd., a pharmaceutical company based in Japan.
Lusutrombopag received its first approval in Japan in September 2015, making it the first country/location to approve the drug. It has also obtained approval in other countries globally. The drug has successfully completed the highest phase of clinical trials and has been granted fast track status, indicating its potential to address an unmet medical need.
As a small molecule drug, Lusutrombopag is designed to interact with the TPO receptor, which plays a crucial role in the production and maturation of platelets. By targeting this receptor, the drug aims to stimulate platelet production and increase platelet count in patients with thrombocytopenia.
Thrombocytopenia is a condition commonly associated with chronic liver disease and is characterized by a decreased number of platelets in the blood. This can lead to an increased risk of bleeding and other complications. Lusutrombopag offers a potential treatment option for patients suffering from thrombocytopenia, providing them with a means to increase their platelet count and reduce the associated risks.
The approval of Lusutrombopag in multiple countries, including Japan, highlights its efficacy and safety profile. The fast track designation further emphasizes the urgent need for effective treatments in this therapeutic area. The drug's mechanism of action, targeting the TPO receptor, demonstrates a targeted approach to addressing thrombocytopenia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for lusutrombopag: TPO receptor agonists
TPO receptor agonists are a type of medication that act on the TPO (thrombopoietin) receptor in the body. Thrombopoietin is a hormone that regulates the production of platelets in the bone marrow. TPO receptor agonists mimic the action of thrombopoietin and stimulate the production of platelets.
From a biomedical perspective, TPO receptor agonists are used in the treatment of certain conditions characterized by low platelet counts, such as immune thrombocytopenia (ITP) and chemotherapy-induced thrombocytopenia. By activating the TPO receptor, these medications promote the production of platelets, which helps to prevent bleeding and improve overall blood clotting function.
TPO receptor agonists can be administered orally or through injection, depending on the specific medication. They are typically prescribed under the guidance of a healthcare professional and require regular monitoring of platelet counts to ensure appropriate dosing.
It's important to note that TPO receptor agonists may have potential side effects, such as headache, fatigue, and nausea. Close monitoring and follow-up with a healthcare provider are necessary to manage any potential risks or complications associated with these medications.
Drug Target R&D Trends for lusutrombopag
The TPO receptor, also known as the thrombopoietin receptor, plays a crucial role in the human body's production of platelets. Thrombopoietin, a hormone produced in the liver and kidneys, binds to the TPO receptor, stimulating the production and maturation of megakaryocytes, which are the precursor cells of platelets. This interaction is essential for maintaining adequate platelet levels in the blood, which are vital for normal blood clotting and preventing excessive bleeding. Understanding the TPO receptor's function has led to the development of drugs that target this receptor, offering potential therapeutic options for conditions associated with low platelet counts, such as immune thrombocytopenia and chemotherapy-induced thrombocytopenia.
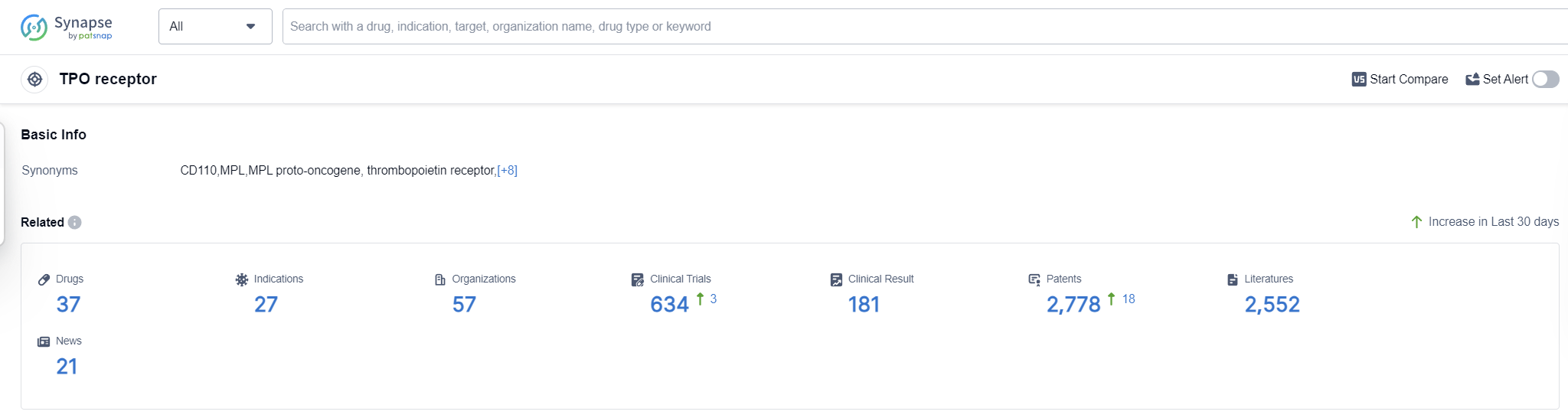
According to Patsnap Synapse, as of 16 Sep 2023, there are a total of 37 TPO receptor drugs worldwide, from 57 organizations, covering 27 indications, and conducting 634 clinical trials.
The analysis of the current competitive landscape of the target TPO receptor reveals that companies such as Kirin Holdings Co., Ltd., Shionogi & Co., Ltd., Amgen, Inc., Eisai Co., Ltd., and Jiangsu Hengrui Pharmaceutical Group Co. Ltd. are leading in terms of R&D progress. The approved drugs primarily target indications such as thrombocytopenia, purpura, thrombocytopenic, idiopathic, and anemia, aplastic. Small molecule drugs, fusion proteins, and biosimilars are the most rapidly progressing drug types, indicating intense competition. China, the United States, Japan, and the European Union are the countries/locations developing fastest, with China showing significant progress. The future development of the target TPO receptor holds promise for the treatment of various indications and the emergence of innovative therapies.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Lusutrombopag is a small molecule drug developed by Shionogi & Co., Ltd. It targets the TPO receptor and is approved for the treatment of thrombocytopenia. The drug has completed the highest phase of clinical trials and has received fast track designation. Its approval in multiple countries, including Japan, underscores its potential as a valuable treatment option for patients with thrombocytopenia.