Exploring the Latest B7-H3 ADC Deal by Hansoh Pharma International Limited: A Guide to Rapidly Accessing Transaction Insights
On December 20, 2023, Hansoh Pharma International Limited and GSK Plc jointly announced that they have entered into an exclusive licensing agreement for Hansoh Pharmaceutical's independently developed ADC (Antibody-Drug Conjugate) new drug HS-20093. Hansoh Pharmaceutical will grant GSK the exclusive rights to develop, produce, and commercialize HS-20093 worldwide, with the exception of Mainland China, Hong Kong, Macau and Taiwan. According to the terms of the agreement, Hansoh Pharmaceutical will receive an upfront payment of $185 million and is eligible to receive up to $1.525 billion in success-based milestone payments. Upon commercialization of the product, GlaxoSmithKline will also pay tiered royalties on worldwide net sales outside of Mainland China, Hong Kong, Macau and Taiwan.
About HS-20093
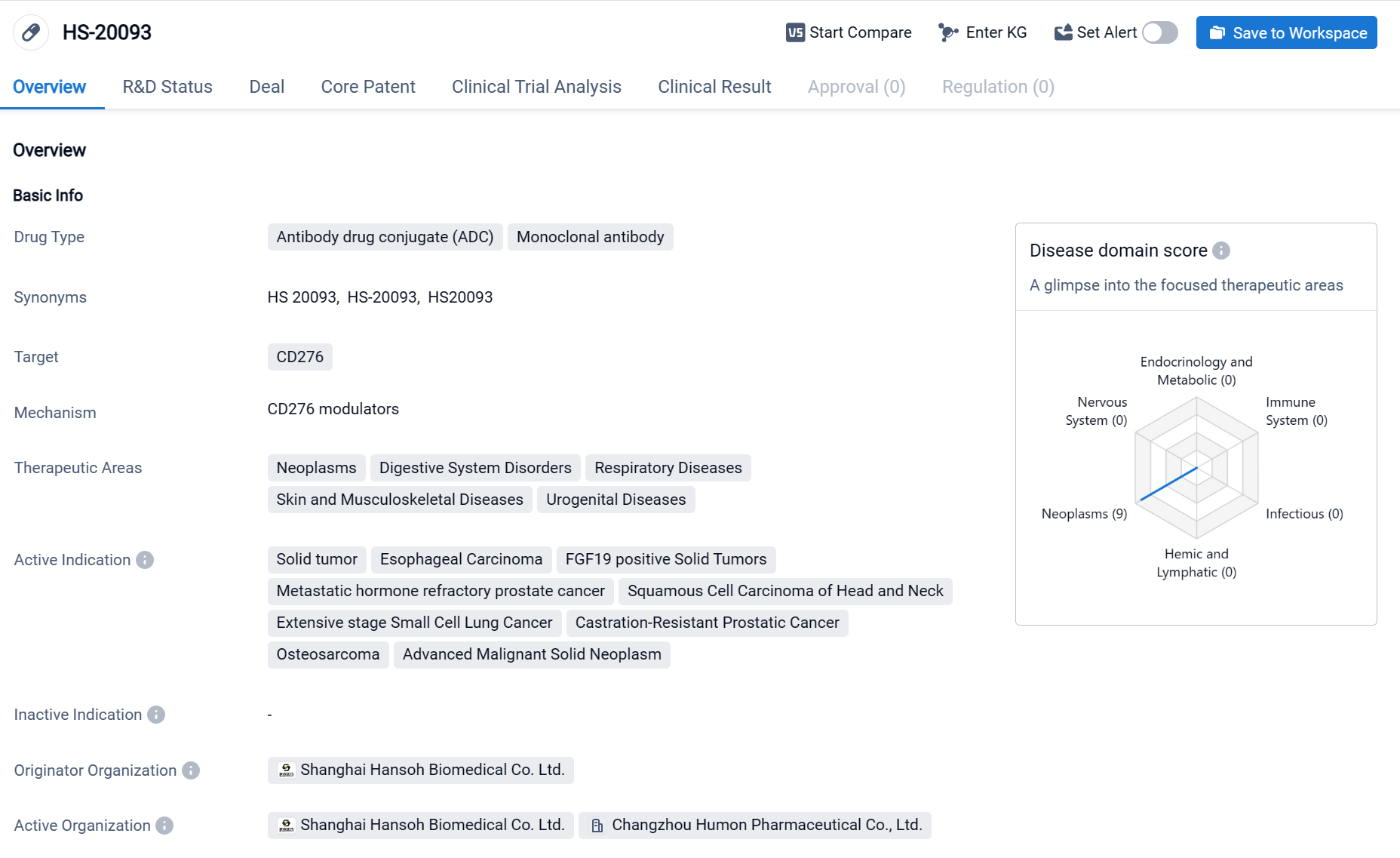
HS-20093 is an antibody drug conjugate (ADC) and monoclonal antibody that targets CD276(B7-H3). It is being developed by Hansoh Biomedical Co. Ltd. and is currently in Phase 2 of clinical trials globally. The therapeutic areas that HS-20093 aims to address include neoplasms, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases. This indicates a broad potential for the drug to be used in various medical conditions. Click the image below to directly embark on the exploration journey with the HS-20093!
Based on the Phase I study data for advanced solid tumors presented at the 2023 ASCO, the results showed that the Objective Response Rate (ORR) was 30.0%, the Disease Control Rate (DCR) was 86.0%, and the median Progression-Free Survival (mPFS) was 5.4 months. Among the small cell lung cancer participants, the ORR was 63.6%, with all tumor responses occurring at the first effectiveness assessment, the mPFS was 4.7 months, and the 3-month PFS rate reached 72.7%. Three subjects experienced dose-limiting toxicity, with the Maximum Tolerated Dose (MTD) being 12.0 mg/kg every 3 weeks (Q3W).
About Hansoh Pharma International Limited
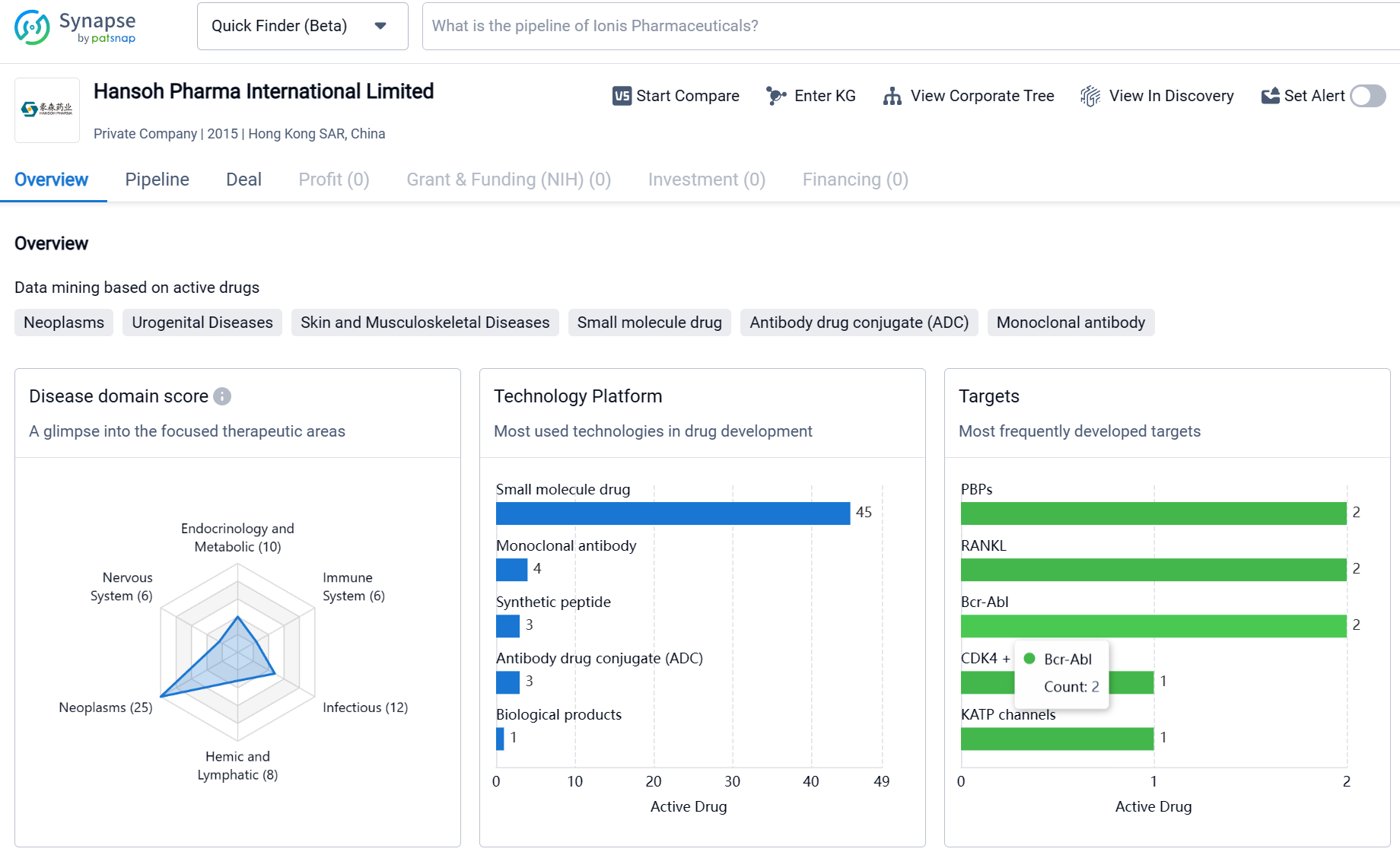
Based in Hong Kong SAR, China, Hansoh Pharma International Limited is a relatively young pharmaceutical organization. Since its establishment in 2015, the company has focused on the development and distribution of drugs for various therapeutic areas. The organization has developed drugs for a wide range of conditions, with a significant emphasis on neoplasms (cancer) and other diseases. The company has also targeted a diverse range of molecular targets, including PBPs, RANKL, Bcr-Abl, and various receptors and enzymes involved in disease pathways. The pipeline of Hansoh Pharma International Limited indicates an active involvement in drug development, with drugs in various stages of clinical development and regulatory processes. Overall, the organization's efforts reflect a commitment to addressing critical medical needs and advancing healthcare in the field of biomedicine.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
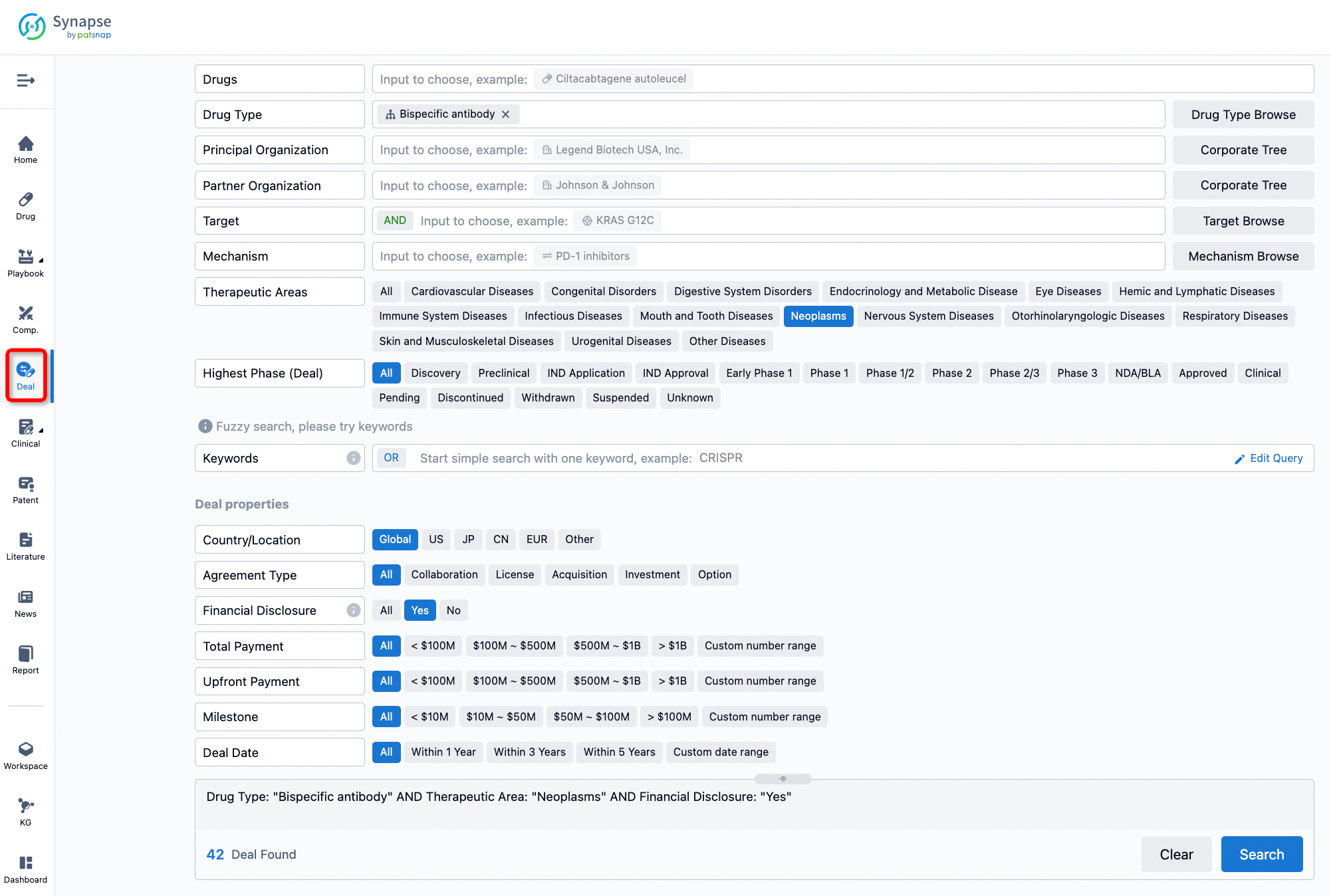
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
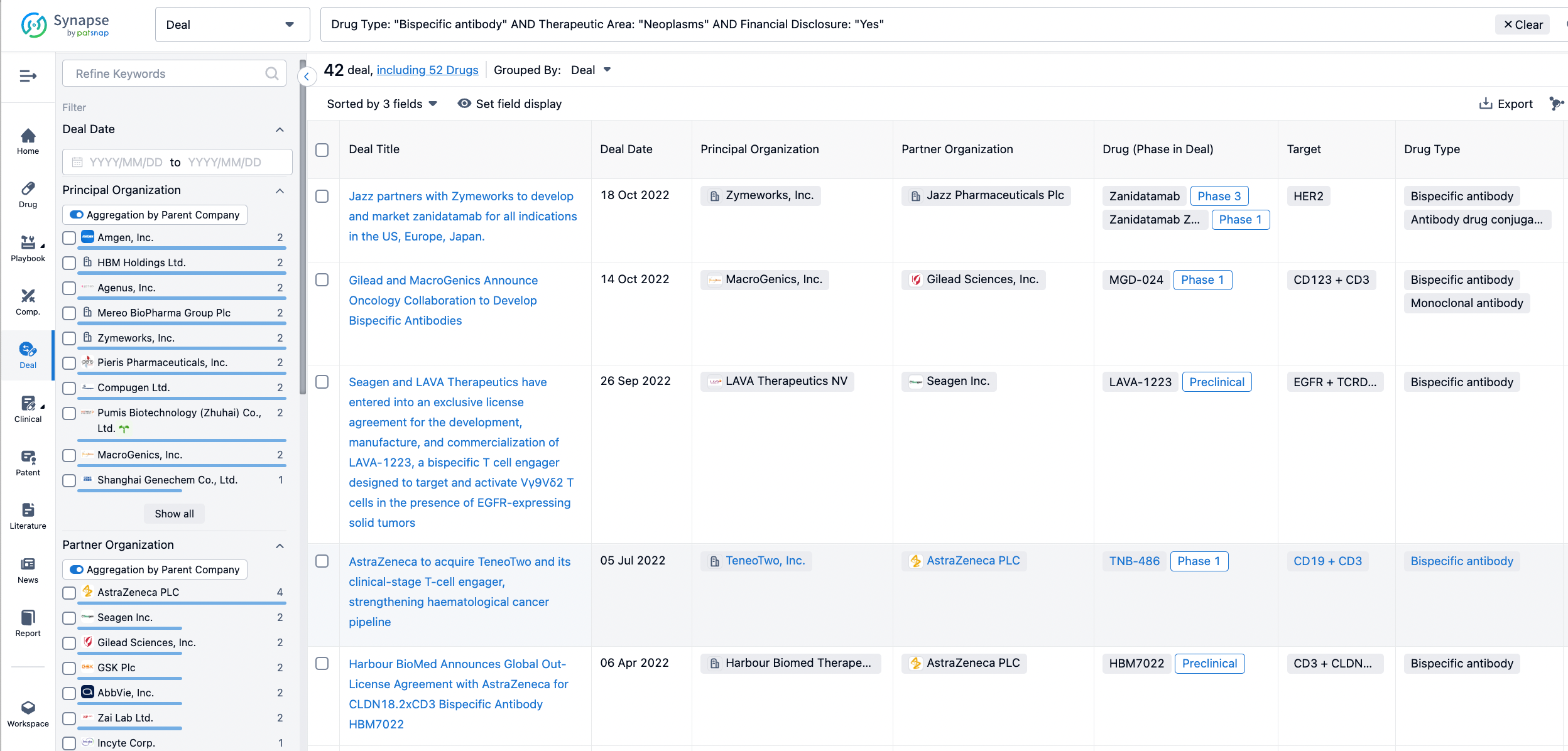
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
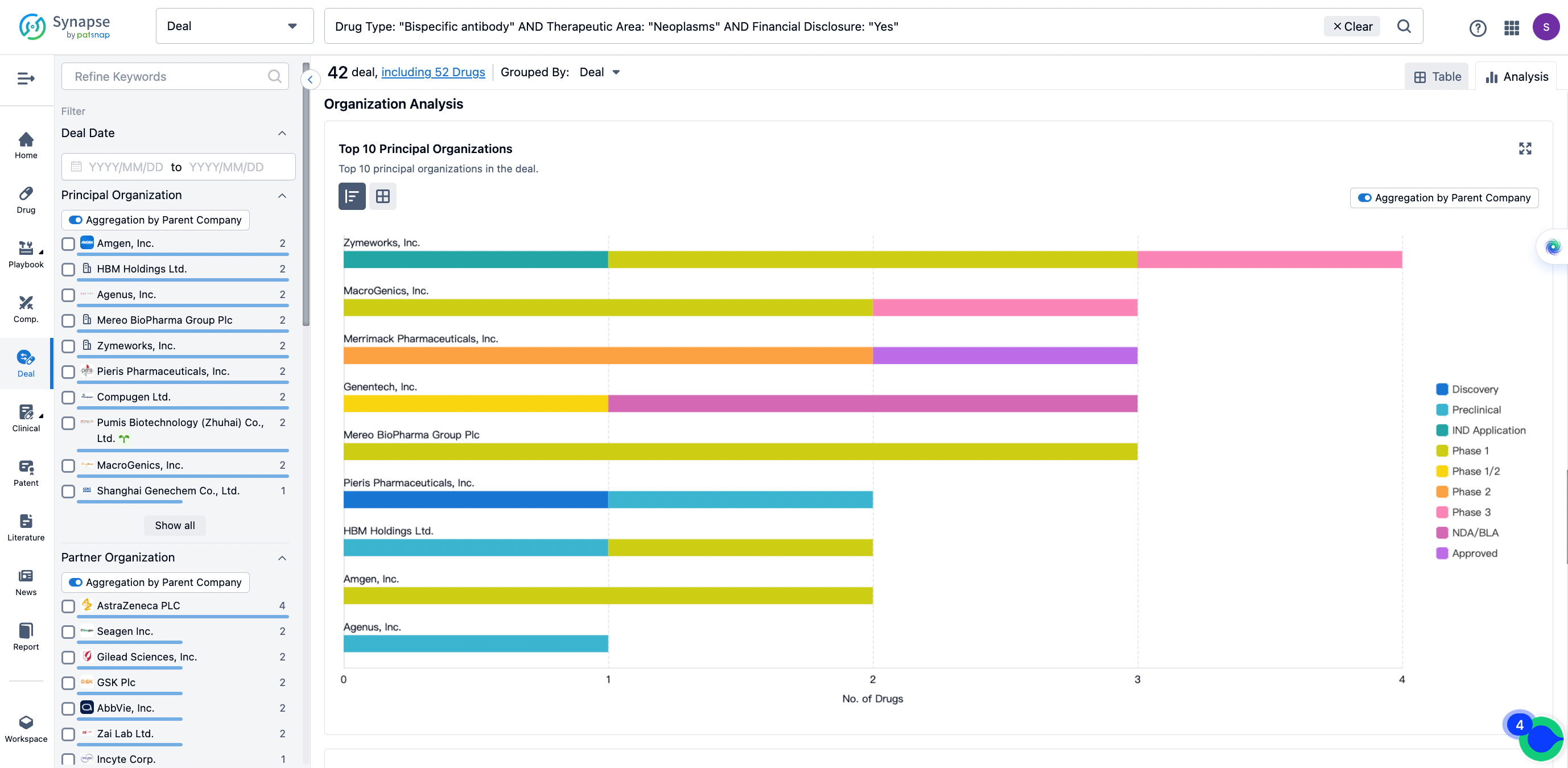
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
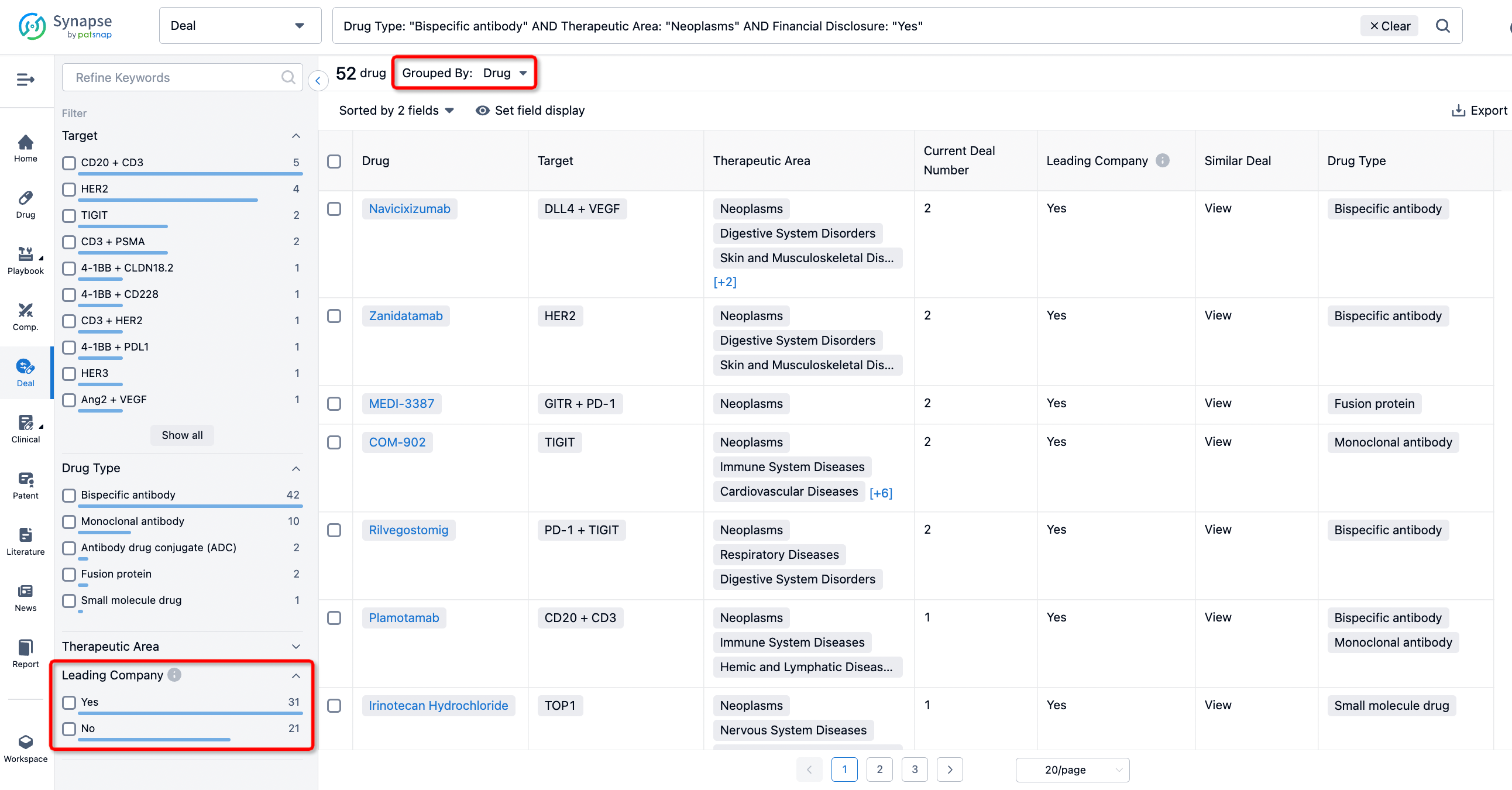
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
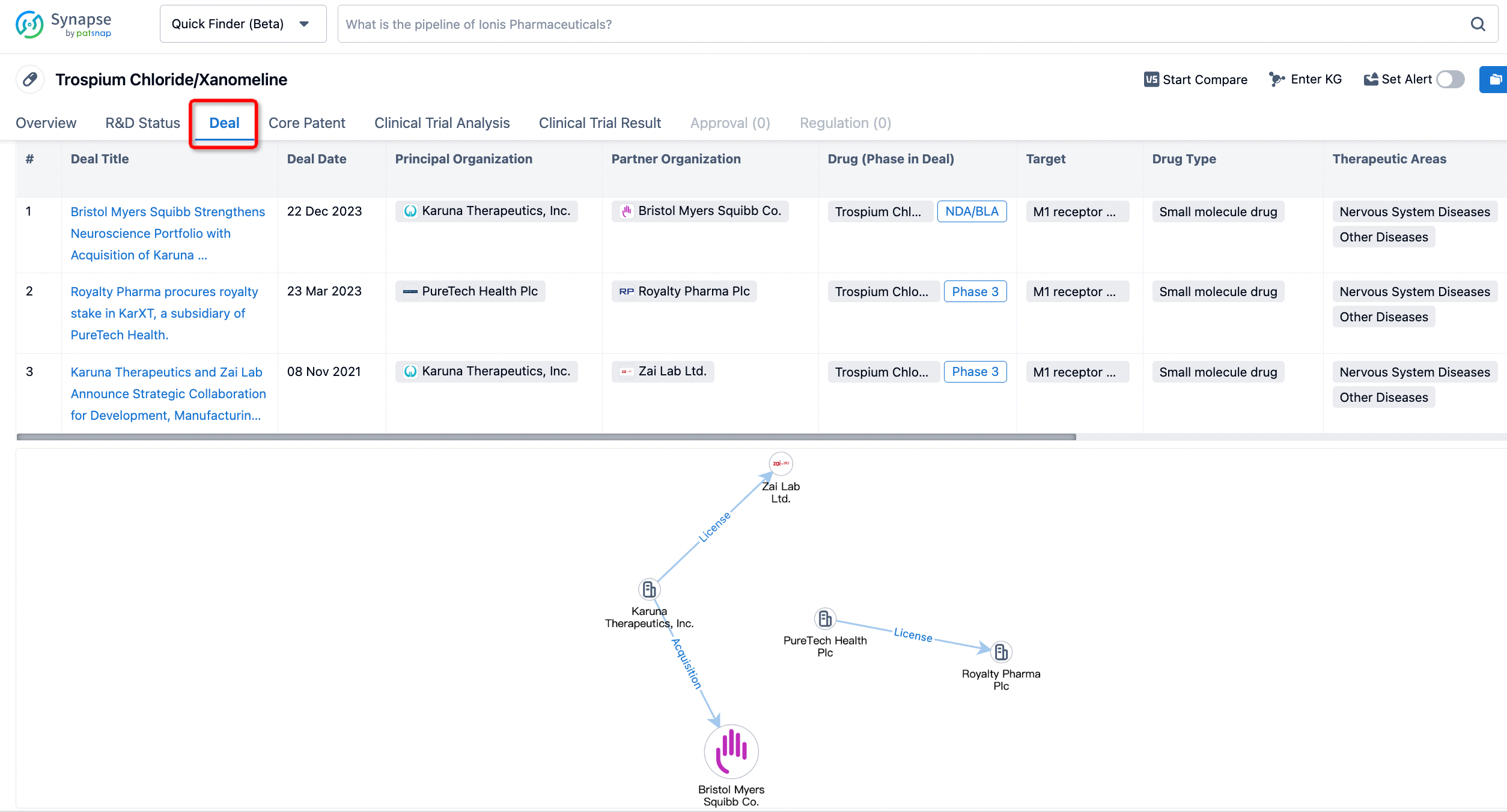
Click on the image below to embark on a brand new journey of drug discovery!