Exploring Tianeptine Sodium's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Tianeptine Sodium's R&D Progress
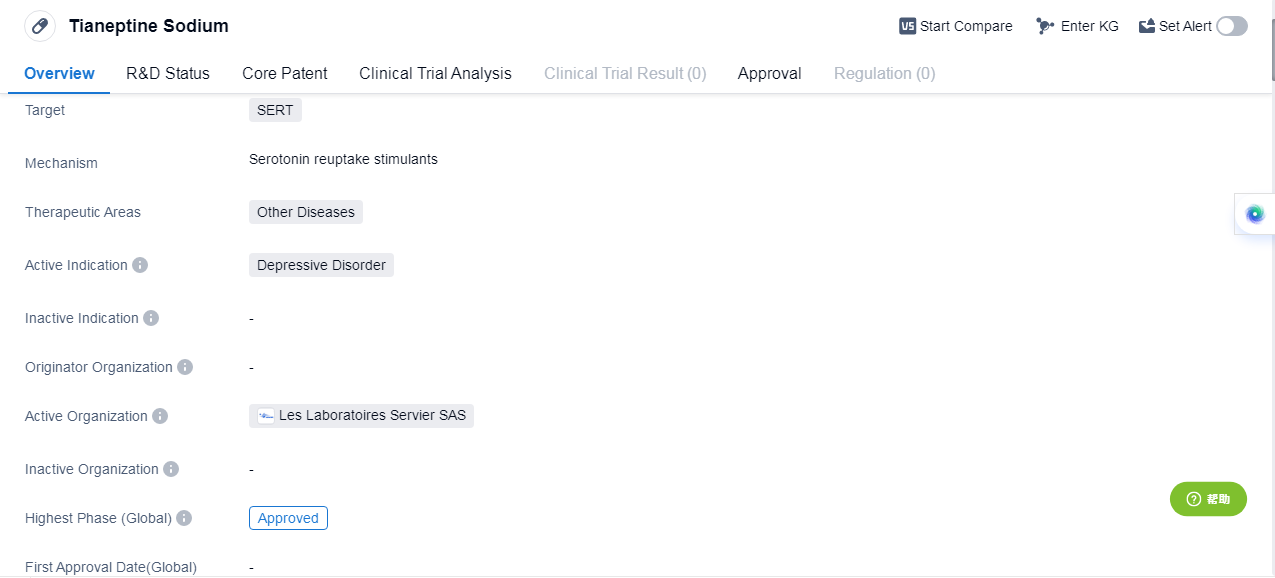
Tianeptine Sodium is a small molecule drug that primari ly targets the serotonin transporter (SERT). It falls under the therapeutic area of Other Diseases and is specifically indicated for the treatment of Depressive Disorder. In terms of its development stage, Tianeptine Sodium has reached the highest phase of approval both globally and in China. This indicates that it has successfully undergone rigorous testing and evaluation, demonstrating its safety and efficacy in treating Depressive Disorder. As a small molecule drug, Tianeptine Sodium is designed to interact with specific molecular targets in the body, in this case, the SERT. By targeting this transporter, the drug is believed to modulate the reuptake of serotonin, a neurotransmitter associated with mood regulation. This mechanism of action is thought to contribute to its therapeutic effects in treating Depressive Disorder. The therapeutic area of Other Diseases suggests that Tianeptine Sodium may have potential applications beyond Depressive Disorder. However, without further information, it is unclear which specific diseases or conditions fall under this category. Further research and clinical trials may be necessary to explore the drug's potential in these areas. The approval of Tianeptine Sodium in global market indicates its recognized safety and efficacy profile. This approval status may open up opportunities for pharmaceutical companies to market and distribute the drug in these regions. Additionally, it suggests that Tianeptine Sodium has met the regulatory requirements and standards set by the respective authorities.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tianeptine Sodium: Serotonin reuptake stimulants
Serotonin reuptake stimulants are a type of medication that work by increasing the levels of serotonin in the brain. Serotonin is a neurotransmitter that plays a crucial role in regulating mood, emotions, and behavior. These stimulants specifically target the reuptake process of serotonin, which is the reabsorption of serotonin by the nerve cells after it has been released into the synaptic gap. By inhibiting the reuptake of serotonin, these medications allow more serotonin to remain in the synaptic gap, enhancing its effects and potentially improving mood and emotional well-being.
From a biomedical perspective, serotonin reuptake stimulants are commonly used in the treatment of various psychiatric disorders, such as depression, anxiety disorders, and obsessive-compulsive disorder (OCD). By increasing serotonin levels, these medications can help alleviate symptoms associated with these conditions. Examples of serotonin reuptake stimulants include selective serotonin reuptake inhibitors (SSRIs) like fluoxetine (Prozac), sertraline (Zoloft), and escitalopram (Lexapro).
It is important to note that the use of serotonin reuptake stimulants should be done under the supervision of a healthcare professional, as they may have potential side effects and interactions with other medications. Additionally, individual response to these medications can vary, and it may take several weeks for their full therapeutic effects to be realized.
Drug Target R&D Trends for Tianeptine Sodium
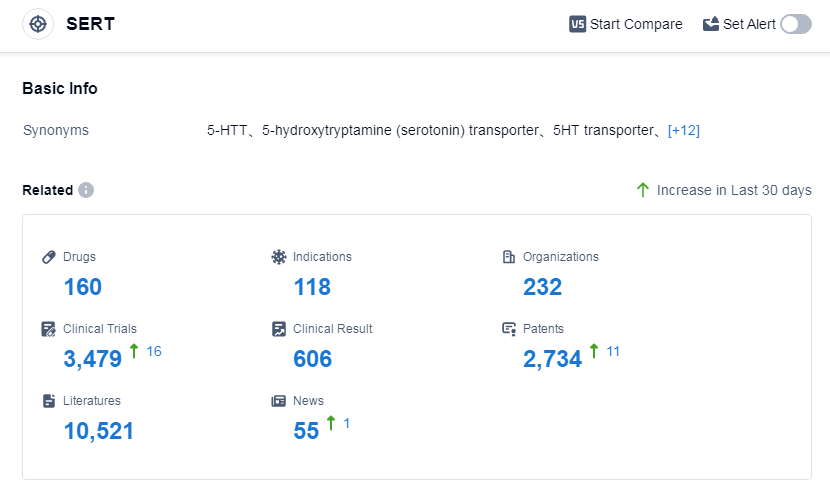
According to Patsnap Synapse, as of 2 Sep 2023, there are a total of 160 SERT drugs worldwide, from 232 organizations, covering 118 indications, and conducting 3479 clinical trials.
The analysis of the target SERT in the pharmaceutical industry reveals a competitive landscape with multiple companies showing growth and progress. AbbVie, Inc., Viatris Inc., Pfizer Inc., Eli Lilly & Co., and Lundbeck Foundation are among the companies growing fastest under the current target. The most common indications for drugs targeting SERT include depressive disorder, depressive disorder major, obsessive-compulsive disorder, panic disorder, anxiety, and social phobia. Small molecule drugs and unknown drug types are progressing rapidly, indicating potential competition and development opportunities. The United States, China, Japan, and the European Union are the countries/locations developing fastest under the current target, with China showing significant progress. Overall, the target SERT presents a promising area for further research and development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Tianeptine Sodium is a small molecule drug that targets the SERT and is indicated for the treatment of Depressive Disorder. It has reached the highest phase of approval globally and in China, indicating its recognized safety and efficacy. While its therapeutic area is categorized as Other Diseases, further research is needed to explore its potential applications in this area. The approval status of Tianeptine Sodium presents opportunities for pharmaceutical companies to market and distribute the drug in the global and Chinese markets.